The Honourable Jean-Yves Duclos, P.C., M.P.
Minister of Health
The Honourable Dr. Carolyn Bennett, P.C., M.P.
Minister for Mental Health and Addictions and Associate Minister of Health
©His Majesty the King in Right of Canada, represented by the President of the President of the Treasury Board, 2023
This document is available on the Treasury Board of Canada Secretariat website.
This document is available in alternative formats upon request.
Table of Contents
From the Ministers
We are pleased to present Health Canada’s 2023-24 Departmental Plan, which outlines the work that the Department will undertake on behalf of Canadians this coming year. The Department’s collaborative work will focus on improving Canada’s healthcare system by prioritizing five fundamental areas.
First, Health Canada will support the transformation of healthcare systems by ensuring Canadians can access the services they need when and where they need them. It will also be supporting health care workers as well as the efforts in reducing backlogs for health services such as surgeries and diagnostics. Our health workers are experiencing unprecedented challenges. Health Canada is committed to advancing collaborative approaches to education, training, licensing, credential recognition and regulation of health professionals. The Department will also continue its work through the Coalition for Action for Health Workers, whose advice will inform immediate and longer-term solutions to address the current health workers crisis.
Secondly, the Department intends to improve access to family health services, including mental health services, which are essential for both preventive health care and for access to effective and equitable health care. This includes improving access to oral health care; to help with these costs, the Government of Canada has introduced the new, interim Canada Dental Benefit, which is the first step in improving access to dental care services for those who need it most. The Government of Canada will continue to work on a comprehensive, long-term Canada-wide dental care program.
Health Canada will also work to ensure that those in long-term care live in dignity, safety and comfort. Federal funding announced in Budget 2021 will help provinces and territories make improvements so that seniors can get the care they deserve. We also know that many Canadians prefer to age at home or in their community, which is why federal funding is being provided until 2027 for provinces and territories to improve access to home and community care. In addition, Health Canada is working with Employment and Social Development Canada on measures such as the Age Well at Home pilot project, which ran this past year, to support Canadian seniors who wish to continue living independently.
Ensuring mental health and substance use services are part of our universal healthcare system is also of paramount importance. Opioid-related overdoses and deaths continue to have devastating effects on people across Canada. Health Canada will continue its federal funding for the Substance Use and Addictions Program, which supports harm reduction, treatment, and prevention at the community level. The Department will also continue to support greater access to services offering safer, pharmaceutical-grade alternatives to the highly toxic illegal drug supply, with the goal of saving lives and reducing drug overdoses.
With input from our partners, the Department will also develop and implement a comprehensive approach to supporting mental wellness that includes national standards for mental health and substance use. The Department will also improve mental health and substance use services that reduce stigma, are culturally appropriate, and address the overdose crisis with innovative and collaborative actions.
Finally, Health Canada is putting digital health and virtual care at the heart of our collaboration with provincial and territorial partners. Making it easier to collect and share health information safely and securely is essential for improving Canada’s healthcare system and health outcomes for Canadians.
These five priorities, however, are not an exhaustive list of the important work Health Canada is undertaking this year.
The Department will continue its work with the Public Health Agency of Canada and other government departments and partners to respond to COVID-19 and intends to maintain its readiness to respond to health emergencies and help build resilience in Canada’s health sector, such as working with Innovation, Science and Economic Development Canada to increase national capacity to develop and produce critical vaccines, therapeutics and other life-saving medicines.
In addition to climate-related health risks, the environment is also top of mind as the Department continues its work to modernize and strengthen the Pest Control Products Act to ensure it supports transparency, increases the use of independent data and stakeholder advice, and modernizes business processes for the decision-making process.
Important work will also continue in the area of pharmacare in the coming year. The Department will continue its collaboration with provinces, territories, and stakeholders to increase access to affordable drugs so Canadians can have the drug coverage they need, when they need it.
To further address and mitigate drug shortages, the Department established a Drug Shortages Task Force in November 2022, which is building upon Health Canada’s work with various stakeholders to safeguard the drug supply. The Department will also continue to increase its regulatory agility and make use of new regulatory tools established during the pandemic, as well as increase its use of data and analytics to monitor and identify potential shortages.
Health Canada looks forward to continuing to work closely with provincial and territorial governments, Indigenous partners, key stakeholders and communities across the country in order to advance priorities that promote and protect the health of all Canadians.
As always, none of the work laid out in this plan would be possible without Health Canada’s employees. Their commitment and dedication are greatly appreciated.
The Honourable Jean-Yves Duclos, P.C. M.P.
Minister of Health
The Honourable Dr. Carolyn Bennett, P.C. M.P.
Minister of Mental Health and Addictions and Associate Minister of Health
Plans at a glance
Health Canada is the federal department responsible for helping Canadians maintain and improve their health. In keeping with the Department’s commitment to making this country’s population among the healthiest in the world, its main responsibilities are as a regulator, a catalyst for innovation, a funder, and an information provider.
Health Canada also administers the Canada Health Act (CHA), which embodies national principles to ensure a universal and equitable publicly-funded healthcare system. In addition to working closely with provincial and territorial (P/T) governments, the Department also works with partners in the Health Portfolio [Public Health Agency of Canada (PHAC), Canada Food Inspection Agency (CFIA), and Canadian Institutes of Health Research (CIHR)], other federal departments and agencies, non-governmental organizations, other countries, Indigenous partnersFootnote 1 and the private sector.
From coast to coast to coast, Health Canada employees – scientists and researchers, inspectors, doctors and nurses, policy analysts and administrative professionals, and many others – are working to help Canadians maintain and improve their health.
Core Responsibilities
Health Canada’s Departmental Results Framework outlines two core responsibilities for the Department: Health Care Systems and Health Protection and Promotion. This reporting framework provides the structure for planned activities, which are organized according to these core responsibilities and their corresponding results.
Core Responsibility 1:
Health Care Systems
Core Responsibility 2:
Health Protection and Promotion
Under the Health Care Systems core responsibility, Health Canada provides national leadership to foster sustainable health care systems that ensure access for Canadians to appropriate and effective health care. This is mainly achieved through partnerships with P/T governments and support through targeted funding agreements to organizations and key pan-Canadian health partners that are contributing to health system improvements.
Within the Health Protection and Promotion core responsibility, Health Canada works with domestic and international partners to assess, manage and communicate the health and safety risks and benefits associated with health and consumer products, food, chemicals, pesticides, environmental factors, tobacco and vaping products, cannabis, and controlled substances. These risks are managed through rigorous regulatory frameworks and by communicating risks and benefits to Canadians so that they can make informed decisions.
Key Plans
In 2023-24, among the many others detailed in this report, Health Canada plans to achieve the following key results that contribute to the health of Canadians.
Core Responsibility 1: Health Care Systems
- Working closely with P/Ts and other stakeholders, Health Canada will continue to modernize Canadian healthcare systems by ensuring Canadians can continue to access the services they need when and where they need them.
- The Department will also deliver on the following five health system priorities:
- Supporting health care workers and reducing wait times by working with P/Ts and other health partners to retain and recruit health workers, including by helping internationally trained health care professionals get their credentials recognized and find work in the healthcare system more quickly, and by improving information about the health workforce so P/T governments can better plan for the future;
- Improving Canadians’ access to family health services, regardless of where they live, by working with P/Ts and other health partners to support and scale-up team-based models that provide care from a team of professionals with different skills, centered on the needs of the patient;
- Working with P/Ts and other health partners toward a modern and connected, world-class health system where Canadians can interact digitally with the health system and access their own health data electronically; where health professionals can share data to improve safety, quality, and care; and, report on how the system is working;
- Improving access to mental health and substance use services by collaborating with a wide range of partners, including access to culturally appropriate services, to counter rising rates of illness and death;
- Helping Canadians age with dignity, closer to home by providing P/Ts with significant investments to address critical long-term care issues by strengthening compliance and enforcement activities and ensuring standards for long-term care are applied and permanent changes are made.
- The Department will continue to provide up to $650 per child to eligible families with children under the age of 12 as part of the interim Canada Dental Benefit so they can get the oral health care their children need while continuing to work on expanding access to dental care services via a long-term Canada-wide dental care program.
- Health Canada will continue to improve access to affordable prescription medicine by working with willing P/Ts towards implementing a national pharmacare program. This includes work to establish a Canadian Drug Agency and a national strategy on drugs for rare diseases. It also includes working with P/Ts to make it easier for Canadians to access and afford medications.
- Health Canada will continue to fund and work closely with the pan-Canadian health organizations to accelerate the modernization and transformation of Canadian health systems.
- In addition, to ensure that Canadians have access to appropriate and effective health services, the Department will focus on: improving access to quality home care, long-term care (LTC) and palliative care; improving access to sexual and reproductive health services; modernizing the interpretation of the CHA; combating cancer; addressing chronic pain priorities in Canada; supporting organ, tissues and blood donation and transplantation; as well as improving equitable access to care–including addressing anti-Indigenous racism in Canada’s health systems.
Core Responsibility 2: Health Protection and Promotion
- Health Canada will continue efforts to provide Canadians with timely access to safe, effective and quality health products, including prescription and non-prescription pharmaceutical drugs, biologic and radiopharmaceutical drugs, natural health products, and medical devices – and to support the needs of the healthcare system.
- In so doing, the Department will focus on: providing timely access to health products; managing and monitoring drug and medical device availability; applying real-world evidence to support regulatory decision-making; strengthening regulatory oversight; as well as acting to prevent and control antimicrobial resistance (AMR).
- To help Canadians lead healthier lives and to provide protection from unsafe consumer and commercial products and substances, Health Canada will focus on: applying a comprehensive approach to substance use-related harms; regulating cannabis; managing the health risks of chemicals in the home, the workplace and the environment; supporting the safety of consumer products and cosmetics; protecting Canadians from radiation; and strengthening pesticide regulation and transparency.
- To help Canadians make healthy choices, the Department will focus on: promoting healthy eating; modernizing the regulatory oversight of food, including its packaging and labelling; ensuring the safety and nutritional quality of the Canadian food supply; taking action on youth vaping and reducing tobacco use; as well as supporting Canadians in making informed decisions about cannabis use through public education, research and surveillance.
Internal Services
- The Department will continue to encourage and support accessibility, diversity and inclusivity through implementing new initiatives set out in the first Health Canada Accessibility Plan, as well as increasing recruitment and representation of persons with disabilities in support of the Accessibility Strategy for the Public Service of Canada.
- Health Canada will focus its internal activities on: building a healthy, diverse and inclusive workforce; nurturing a respectful, diverse, and inclusive work environment by providing a safe space where employees can raise and discuss work-related concerns and find options, resources, and tools to address them; enabling a safe and productive workforce with access to modern tools and facilities; as well as providing Canadians with inclusive, timely and evidence-based information needed to take action on their personal and collective health and safety.
Sex- and Gender-Based Analysis Plus (SGBA Plus/GBA Plus)
In 2023-24, Health Canada will continue to implement its Sex- and Gender-Based Analysis Plus (SGBA Plus) Action Plan, through the following initiatives: increasing governance, accountability and transparency in the integration of SGBA Plus in the Department’s decision-making; enhancing Departmental knowledge and capacity to apply SGBA Plus using an intersectional lens and a deeper understanding of systemic issues to advance equity, diversity and inclusion in the work of the Department; collaborating with internal and external partners to strengthen the Department’s sex-, gender- and diversity-related evidence base and expertise; enabling the collection and use of disaggregated data for rigour in intersectional analysis; and enhancing communications, guidelines, tools and resources with clarity on SGBA Plus and intersectionality.
The United Nations 2030 Agenda for Sustainable Development and UN Sustainable Development Goals (SDGs)
As part of Canada’s commitment to support the United Nations 2030 Agenda for Sustainable Development and UN Sustainable Development Goals, Health Canada’s domestic contribution through ongoing policies, programs and initiatives advances five SDGs: good health and well-being (3); clean water and sanitation (6); sustainable cities and communities (11); responsible consumption and production (12); and climate action (13).
Innovation
Health Canada will continue to increase employee capacity for innovation. Planned initiatives include: Project Apollo, Project Cognit.IO, Project Heart, and Project D.A.T.A. (Data Annotation Training sets for Artificial Intelligent Tools). Additional innovation and experimentation initiatives that will begin or continue in 2023-24 include: Project Citizen Science, Project Kelpie, Project GenomicsCompTox Cloud Computing, and the Chemicals Management Plan Contribution Program (Indigenous Funding Stream).
For more information on Health Canada’s plans, priorities and results achieved, see the “Core responsibilities: planned results and resources, and key risks” section of this plan.
Planned results and resources, and key risks
Core Responsibility 1: Health Care Systems
Description
Health Canada provides national leadership to support and encourage sustainable and adaptable healthcare systems that ensures access for Canadians to appropriate and effective health care services.
Planning Highlights
Modern and sustainable healthcare systems are vital to addressing the health needs of Canadians. Although health care delivery is primarily under P/T jurisdiction, the Government of Canada (GOC) plays an important role in a range of health care and health system priorities. This includes supporting the modernization of the healthcare system so that it can meet a broader range of needs, with a focus on dental care, affordable pharmaceuticals, mental health and substance use services and modern health data and digital systems that provide better connected care. As part of this work, the GOC catalyzes and encourages innovation in service delivery to meet the needs of patients, families and communities. Health Canada also administers the CHA and upholds its core principles which underpin the publicly funded health systems in Canada.
Health systems continue to evolve in a context of technological and social changes and in the face of global health challenges, such as the COVID-19 pandemic and its negative impact on Canadians’ timely access to care and subsequent growth of backlogs. The Department will continue to play a leadership role to improve the quality and sustainability of Canada’s healthcare systems. This will ensure that Canadians can continue to access the services they need when and where they need them.
Health Canada will continue to work with P/Ts and other partners to transform Canadian health systems and deliver on the following health priorities:
- Supporting health care workers and reducing wait times – Increase the supply of health care workers and create healthier workplaces to support retention and mental health by through initiatives such as helping internationally trained health care professionals get their credentials recognized in Canada and find work in the healthcare system. This will help to increase the number of doctors and nurses in communities that need them the most. The GOC is providing an additional $6.5 billion in top-ups to the Canada Health Transfer to help address the diagnostic and surgical backlogs in our hospitals and reduce wait times caused by the pandemic.
- Improving Canadians’ access to family health services – Improve access to family health services across the country, with a focus on a range of different professionals supporting patients, as well as enabling health care providers to work in underserved rural and remote communities.
- Working towards a modern, connected, world-class health system – Improve how health data is collected and used to better inform the care that patients receive. This, in conjunction with improved digital health systems, will allow Canadians to access their own health information in a timely manner.
- Improving access to mental health and substance use services – Collaborate with a wide range of partners to ensure Canadians can access culturally appropriate mental health and substance use services to counter rising rates of illness and death due to these issues. Through the Wellness Together Canada portal, and its companion app PocketWell, the Department continues to provide a range of free, self-guided resources and supports in both official languages.
- Helping Canadians age with dignity, closer to home – Support Canadians who wish to age at home and in their community with appropriate home care and LTC services. Health Canada will work with P/Ts to ensure seniors get the care they deserve. This includes supporting P/Ts by providing significant investments to address critical long-term care issues, such as compliance and enforcement and staffing. Federal funding to P/Ts for these needs includes $6 billion over ten years, starting in 2017 to increase home and community services, and $3 billion from Budget 2021 to ensure standards for long-term care are applied and permanent changes are made.
In addition, the Department will continue to administer the CHA and assess new and emerging issues, with the aim of working with partners to further strengthen the resilience of Canadian health systems.
Departmental Result 1: Canadians have modern and sustainable health care systems
Health Canada works closely with P/Ts, domestic and international organizations, health care providers and other stakeholders to develop and implement innovative approaches that improve the efficiency, effectiveness and sustainability of Canadian healthcare systems. The Department also conducts research and provides evidence-based policy advice in support of modernizing the healthcare system.
In 2023-24, the Department will continue to work with P/Ts to strengthen publicly funded health services in Canada, with a focus on expanding access to dental care services. Further, Health Canada will continue to fund community and other third party organizations to test new approaches to improve health services delivery. Priorities include palliative and end-of-life care, home and community care, mental health and substance use support, including the overdose crisis, primary care, addressing chronic pain priorities, digital/virtual care, and health human resources.
Additionally, Health Canada will consider and apply lessons learned from the COVID-19 pandemic to its programs and activities. In 2023-24, the Department will establish a health emergency coordination office to maintain readiness for health emergencies. This office will include a centralized secretariat as the Departmental point of contact for emergency management working with federal partners, P/Ts and other stakeholders.
The Department will also continue to work with Innovation, Science and Economic Development Canada to advance the Biomanufacturing and Life Sciences Strategy as announced in June 2021. The Strategy aims to increase national capacity to manufacture critical vaccines, therapeutics, and other life-saving medicines. Efforts will focus on growing a strong and competitive domestic life sciences sector, ensuring Canada’s readiness for future pandemics or other health emergencies.
Expanding access to dental care services
As part of Health Canada’s efforts to improve access to health services, which are essential for preventative, effective and equitable health care, the Department will expand access to oral health care, which is an important element of overall health. Poor oral health can lead to a wide range of diseases, including cancer, cardiovascular disease, and life-threatening infections, and can have a significant negative impact on quality of life, especially during formative childhood years. There are over 2 million missed school days annually due to toothaches or tooth decay. In addition, poor oral health in Canada is estimated to cause productivity losses of over $1 billion each year and can also increase public spending in other cost-intensive areas of health care, including cardiac, cancer, and emergency services.
Some Canadian families struggle with the costs of dental care. In response, Budget 2022 invested $5.3 billion over 5 years, with $1.7 billion ongoing, to provide dental care for Canadians with family incomes below $90,000.
The interim Canada Dental Benefit, launched in December 2022 in partnership with the Canada Revenue Agency (CRA), is available to children under the age of 12 in families who do not have access to dental insurance to help them get the oral health care they need. Communication and outreach activities will continue in 2023-24 to ensure eligible families are aware of and know how to access the interim Canada Dental Benefit, which will be available until 2024. Health Canada and the CRA are collaborating closely on an application platform that would deliver payments in a timely fashion.
This is the first step towards fulfilling the GOC’s commitment to develop a long-term, Canada-wide Dental Care Program for uninsured individuals within the targeted income range. In parallel, the Department will continue to take important steps to support the development and launch of the long-term Canada-wide Dental Care Program, which will help to remove barriers to accessing needed dental care and support Canadians in improving their oral health. Health Canada will continue to foster relationships and undertake engagement with P/Ts, as well as key stakeholders to inform implementation of the program.
Supporting the modernization of Canadian Health Systems
In 2023-24, Health Canada will continue to work closely with the pan-Canadian health organizations to accelerate modernizing and transforming Canadian health systems. Specifically, the Department will help modernize health data and digital systems through funding to Canada Health Infoway and the Canadian Institute for Health Information (CIHI). It will also advance its work with the Canadian Agency for Drugs and Technologies in Health (CADTH) on the cost effectiveness of drugs and therapies, with Healthcare Excellence Canada (HEC) on system innovations, and with the Brain Canada Foundation to support brain research.
Priorities include:
- Advancing interoperability of digital health solutions – The Department, through Canada Health Infoway, will support P/Ts to advance digital health information for Canadians, e-prescribing, and the ongoing implementation of a pan-Canadian data and performance reporting system for Organ Donation and Transplantation.
- Accelerating improvements in health care, health system performance and population health across the continuum of care – The Department has earmarked $107.7 million for the CIHI to enable them to close data gaps in priority areas such as mental health and substance use services, organ donation and transplantation, as well as the health workforce.
- Strengthening the effective management of drugs and non-drug technologies – Health Canada will provide the CADTH with $37.8 million to support health technology assessments and generate evidence on the effectiveness of drugs and non-drug health technologies to support decision makers, such as public drug plans and health care practitioners.
- Encouraging innovation and catalyzing change to support large-scale health systems improvement – HEC will receive $25.1 million to continue to expand their efforts on three key priorities: re-imagining care with and for older adults with health and social needs; providing care closer to home and community with safe transitions; and contributing to pandemic recovery and health system resilience.
- Supporting brain research – Health Canada has pledged to match private and charitable contributions raised by Brain Canada Foundation up to $200 million by 2024-25. As a result, in 2023-24, the Department will provide $23.4 million to improve the diagnosis, treatment and prevention of brain health conditions through support for innovative neuroscience research.
These efforts will enable governments, health care providers, stakeholders, patients and individual Canadians to have access to better information on the performance of Canadian health systems. They will have further information as to the cost-effectiveness of drugs and technologies to support evidence-based decision-making and P/Ts will have a pool of successful innovations and experiments from which to adapt and scale in support of their efforts to improve health services within their jurisdictions.
Departmental Result 2: Canadians have access to appropriate and effective health services
Through Health Canada, the GOC is responsible for promoting and defending the core principles of the CHA – public administration, comprehensiveness, universality, portability and accessibility – and ensuring that P/T health care insurance plans provide reasonable access to health services without financial or other barriers, such as patient charges for insured services.
The GOC provides financial contributions to P/Ts to support publicly funded health care services through the Canada Health Transfer (more than $43.1 billion for 2022-23). Health Canada also provides targeted funding in key areas such as supporting improved access to mental health and substance use services and home and community care (i.e., $11 billion over 10 years starting in 2017 with $6 billion to improve home and community care and $5 billion for mental health and addiction services). Following agreement by F/P/T Ministers (except Quebec) on a Common Statement of Principles on Shared Health Priorities in 2017, the GOC negotiated and signed bilateral agreements with each P/T, setting out details of how each jurisdiction will use federal funding to improve access to home and community care and mental health and substance use services. Agreements for the first five years expired March 31, 2022, and discussions are underway with P/Ts to renew these agreements for the remaining years.
In 2023-24, to ensure that Canadians have access to appropriate and effective health services, Health Canada will focus on: supporting health human resources; working towards national universal pharmacare; expanding access to mental health and substance use services – including advancing a comprehensive strategy to address substance use in Canada; addressing chronic pain priorities in Canada; improving access to quality home care, LTC and palliative care; supporting primary and virtual care and advancing digital health and health data in collaboration with P/Ts; supporting safe and consistent implementation of medical assistance in dying (MAID); improving access to sexual and reproductive health services; modernizing the interpretation of (and strengthening compliance with) the CHA – including encouraging P/T compliance with the diagnostic services policy; combating cancer; supporting organ, tissues and blood donation and transplantation; as well as improving equitable access to care–including addressing anti-Indigenous racism in Canada’s health systems.
Supporting health human resources
Health care workers are fundamental to ensuring the health and well-being of Canadians. They provide treatment, services and advice and can include physicians, dental hygienists, nurses, and pharmacists, among others. Canada’s health workforce is currently facing challenges. There is a low supply of health workers and retention rates are low, due in part to workplace conditions that put additional pressure on workers. High patient workloads and fewer workers have led to unprecedented levels of burnout, absences and turnover. Vacancy rates and staffing shortages continue to grow as increasing numbers of health care professionals choose to leave their jobs due to difficult working conditions, workplace stress, and challenges in maintaining a healthy work-life balance.
What’s New?
Health Canada has taken steps to address key concerns voiced by health care providers including:
- Convened a stakeholder symposium in spring 2022 to better understand the challenges facing the health workforce;
- Announced in August 2022, Dr. Leigh Chapman as the federal Chief Nursing Officer for Canada to look at nursing issues from a federal focus and provide strategic advice to Health Canada;
- Established a Coalition for Action for Health Workers which will inform immediate and longer-term solutions to address significant health workforce challenges.
The federal government provides financial support to P/Ts for health care services. In July 2022, the GOC transferred $2 billion to P/Ts to support them in hiring new family doctors, nurses and nurse practitioners, and to reduce backlogs in services that are delaying access to medically necessary care. Health Canada is committed to working with P/Ts, health system partners and stakeholders to address the above challenges, including developing sustainable solutions to support and bolster the health care workforce. This includes:
- Taking an active role, along with other federal departments, P/Ts and key stakeholders, in identifying immediate and long-term solutions to address health workforce needs;
- Focusing on priorities such as retention and recruitment, including supporting the integration of internationally educated health professionals;
- Equipping decision-makers with the data and tools needed to enable better planning and innovative strategies, such as collaborating with CIHI and Statistics Canada to effectively collect, analyze and distribute workforce data to support decision-making;
- Expanding partnerships with other stakeholders, such as professional associations, unions, and universities and colleges, among others;
- Driving systemic change within the health system through the Coalition for Action for Health Workers, which will help implement initiatives that support retention of health workers, increase the supply of health professionals in the country through enhanced education and training, and build on opportunities to scale new models of care to address key barriers;
- Continuing to promote the workforce’s mental health and wellness via the tools available through the Wellness Together Canada portal and its companion PocketWell app.
Working towards national universal pharmacare
Since April 2021, Health Canada has engaged extensively with P/Ts, stakeholders, representatives from Indigenous communities, patient advocates and international leaders in pharmaceuticals management on the potential scope and functions of a Canada Drug Agency (CDA). Pharmaceutical system gaps identified include inadequate infrastructure, poor return on investment and fragmentation. In 2023-24, the Department will begin developing the core functions of the CDA, including opportunities to take a coordinated approach to addressing these gaps, building on P/T successes. The GOC also plans to introduce a bill to create a Canada Pharmacare Act by the end of 2023.
The Department will continue to work with the province of Prince Edward Island to support their efforts to provide residents with more access to affordable prescription drugs by allocating federal funding of $35 million over four years (from 2021-22 to 2024-25) and under the Improving Affordable Access to Prescriptions Drugs Program. Building on significant progress to date, which has enabled many PEI residents to access more affordable treatments for cancer, heart disease, migraine, and mental health, the Government of PEI is aiming to make further improvements to their drug programs, such as increasing coverage and simplifying access.
Health Canada will continue to engage with all willing P/Ts to implement a National Strategy on Drugs for Rare Diseases to ensure Canadians with rare diseases have access to the drugs they need. The Strategy will also aim to broaden treatments and services for rare diseases and build national governance with a supporting data infrastructure, and invest in critical research in the area of rare diseases.
Expanding access to mental health and substance use services
In 2023-24, Health Canada will continue working with partners and stakeholders to develop and implement a comprehensive, evidence-based mental health plan. This plan aims to improve access to quality and timely mental health services and ensure that mental health care is treated as a full and equal part of the universal healthcare system. It will also consider and address health inequities, including for Indigenous Peoples, Black Canadians, and other priority populations. The Department will also continue to support projects that promote mental health and prevent mental illness among populations disproportionately affected by the pandemic, with a Budget 2022 commitment of $100 million.
The Department will also continue to work with partners and stakeholders to develop national standards for mental health and substance use services. These standards will support P/Ts, health organizations and other key stakeholders in their efforts to provide high-quality and equitable care for Canadians. The Department will also continue examining expanding access to community-based mental health and substance use services for children and youth.
Further, Health Canada will provide $14.25 million in 2023-24 to the Mental Health Commission of Canada to advance priorities in the areas of suicide prevention, the integration of mental health and substance use, population-based initiatives, and engagement. The organization will also continue to provide mental health education and training, including for targeted populations, to promote mental health and address stigma.
Did you know?
In 2023-24, Health Canada will support P/Ts reporting on their use of a one-time $150 million emergency fund established in Budget 2018 to address the opioid overdose crisis. Since 2018, this initiative has resulted in improved access to evidence-based treatment services, including reduced treatment wait times in some regions, increased numbers of treatment beds, enhanced youth services, and improved access to culturally appropriate care for Indigenous communities.
Maintaining access to virtual health services with Wellness Together Canada
Virtual delivery of mental health and substance use care helps improve timely access to supports and services. Health Canada’s Wellness Together Canada portal will continue to provide quality and timely mental health and substance use supports online, and via text and phone. Building on the commitment from Budget 2022, starting in 2022-23, the Department will invest $140 million over two years for the Wellness Together Canada portal so it can continue to provide Canadians with tools and services to support their mental health and well-being.
As of November 2022, more than 2.9 million individuals across Canada had accessed the portal, and data shows that users experience a positive change on self-assessment scales after using the portal’s support services. PocketWell, the free companion app to the portal, was launched in January 2022 and has since been downloaded more than 30,000 times. The app provides another way to help Canadians access mental health and substance use resources and supports, and to measure and track aspects of their mental well-being.
In 2023-24, Wellness Together Canada representatives will reach out to various populations, focusing on Indigenous communities, organizations serving Black and diverse communities, veterans, and health care workers. This will help to improve access for a more diverse audience and improve the resources, supports and services provided via the portal.
Addressing chronic pain priorities in Canada
In March 2021, the Canadian Pain Task Force provided its final report to Health Canada – An Action Plan for Pain in Canada – with recommendations to ensure that people with pain are recognized and supported and that pain is understood, prevented, and effectively treated throughout Canada.
In 2023-24, Health Canada will continue to coordinate federal efforts to respond to priorities identified by the Task Force. The Department will also continue to work with other federal departments and agencies, P/Ts, pain experts and people living with pain, to facilitate the dissemination of best practices and identify opportunities to advance Task Force recommendations. Health Canada will continue to invest up to $4.5 million over 5 years towards Pain Canada – a national organization dedicated to coordinate efforts and create new opportunities for action by connecting people, ideas, organizations and resources from across the country.
Improving access to quality home care, long-term care and palliative care
Home care
The GOC is committed to supporting programs and services that seniors need, should they want to live independently, in their homes and with the communities that support them, for as long as possible. As announced by the GOC in October 2022, the National Seniors Council will serve as an expert panel to examine measures, including a potential aging at home benefit, to further support Canadians who wish to age in the comfort of their own homes.
Did you know?
Comprised of members with experience or expertise on seniors and aging issues, the National Seniors Council engages with seniors, stakeholders and experts to provide advice to the GOC on issues and opportunities related to the health, well-being and quality of life of seniors. Since its inception in 2007, the Council has examined and provided recommendations on issues such as social isolation, labour force participation, volunteering, low income among seniors, elder abuse, and most recently, issues emerging from the COVID-19 pandemic.
Long-term care (LTC)
The COVID-19 pandemic disproportionately affected Canadians receiving LTC in community settings, such as seniors’ residences and other settings that provide care for seniors. In 2023-24, Health Canada will continue to invest $3 billion over 5 years (2022-23 to 2026-27, $600 million per year), as outlined in the Fall 2022 Economic Statement to help P/Ts and health care organizations make permanent improvements in the standard of care in their LTC facilities, including improving workplace stability and quality and safety improvements. On December 1, 2022, the Canadian Standards Assocation Group posted their new National Standard for long-term care homes operations and infection prevention and control, and on January 31, 2023, the Health Standards Organization released their new National Long-term Care Services Standard, which addresses the delivery of safe, reliable, and high-quality long-term care services. Federal funding announced in Budget 2021 will help P/Ts make improvements in line with these recently announced long-term care standards by strengthening compliance and enforcement activities, including through accreditation and regular inspections, and supporting workforce stability through wage top-ups and improvements to workplace.
Palliative care
Budget 2021 provided $29.8 million over 6 years (2021-22 to 2026-27) to advance the GOC’s Action Plan on Palliative Care, including initiatives aimed at: raising awareness of the importance of palliative care; providing public education on grief; improving palliative care skills and supports for health care providers, families, caregivers, and communities; enhancing data collection and research; and improving access to culturally sensitive palliative and end-of-life care.
In 2023-24 Health Canada will: continue the multi-year awareness campaign for palliative care to raise awareness of its benefits and increase grief literacy; support models of care to underserved populations, including the homeless and vulnerably housed; engage with Indigenous organizations on developing a distinctions-based frameworks for palliative care; and produce a report on the state of palliative care to be tabled in Parliament by December 2023.
Supporting primary and virtual care and advancing digital health and health data
Budget 2022 reiterated the GOC’s commitment to work with P/Ts to enhance access to family health services. Health Canda will continue to explore new approaches to support P/Ts, including the hiring of new primary health care providers, as well as implementing new service delivery models, and digital and virtual care solutions.
Health Canada will continue discussions with P/Ts on how to support greater patient-provider attachment in primary care, enabled by virtual care and digital health. Specific focus will be applied to overcoming gaps in rural and underserved communities, as well as encouraging innovative approaches to care delivery that support providers in working together as interdisciplinary teams to provide comprehensive care to Canadians. This includes supporting researchers and other partners seeking to engage Canadians on the future of team-based primary care in Canada. To address gaps in family health services, the GOC is increasing the maximum amount of forgivable Canada Student Loans for doctors and nurses who work in underserved rural and remote areas by 50%. This will mean up to $30,000 in loan forgiveness for nurses and up to $60,000 for doctors working in these communities.
Appropriate use of virtual care is also a key enabler of improved access to family health services. Virtual care and digital health tools allow Canadians to receive health care services more efficiently, often directly from their homes. The GOC is working closely with P/Ts to expand virtual health services so that Canadians can continue to access the care they need when they need it. Building on the $150 million that was provided to P/Ts over the past two years to support virtual care and digital tools, the Department will continue to collaborate closely with P/Ts to drive pan-Canadian collaboration for supporting the appropriate use of virtual care and digital health within Canadian health systems. Health Canada will also continue collaboration with experts and stakeholders to share learning and best practices on equitable adoption of virtual care and digital health solutions, including supports for providers to help them effectively leverage virtual care within their practice.
Likewise, health data is essential for smooth and seamless health care delivery and can support health system improvements. However, too often health data is trapped in digital systems, unable to be exchanged between providers or accessed by patients due to inconsistent standards. Recognizing this, F/P/T governments have been working together to help ensure health data can flow seamlessly between health data systems, with Infoway leading the development of a pan-Canadian interoperability roadmap, in collaboration with CIHI, Statistics Canada, P/Ts, vendors and other key stakeholders.
Further, building on what was learned during the pandemic, in the fall of 2020, Health Canada, together with P/Ts, began developing a Pan-Canadian Health Data Strategy to improve Canada’s collection, sharing and use of health data. The Department will continue to work directly with P/Ts to advance shared digital health and health data priorities to ensure our health system is underpinned by health data that will support healthcare system improvements so that Canadians can access their personal health records.
Supporting safe and consistent implementation of medical assistance in dying (MAID)
Health Canada continues to support the implementation of MAID legislation. In 2023-24, activities will include:
- Implementing the Regulations for the Monitoring of MAID, including advising practitioners and P/Ts of changes in data collection and reporting;
- Developing policies to implement key recommendations of the independent Expert Panel on MAID and Mental Illness’ review of MAID for persons suffering solely from mental disorders;
- Supporting the mandated Parliamentary review of the MAID legislation;
- Ongoing support to develop and launch a national accredited training curriculum to support practitioners in their assessment and delivery of MAID;
- Implementing a multi-year research program to guide policy work around the evolution of MAID in Canada;
- Ongoing data collection and analysis, including the release of the fourth federal Annual Report on MAID in Canada.
Improving access to sexual and reproductive health services
Health Canada is supporting community-based organizations that help make sexual and reproductive health services more accessible for underserved populations, such as 2SLGBTQI+ populations, Indigenous peoples, racialized people, and youth. From the $45 million provided by Budget 2021 over 3 years (2021-22 to 2023-24), the Department has invested $15.3 million to two organizations supporting access to abortion and seven organizations supporting access to quality health care for 2SLGBTQI+ populations and youth. A further $9.7 million has been allocated to the government of Quebec to invest in community organizations within the province. Based on the 2022-23 call for proposals, Health Canada will invest the remaining $18.3 million in new projects addressing topics such as: sexual and reproductive health resources for Indigenous peoples; supporting health care providers in providing abortion care, and providing culturally safe sexual and reproductive health care for Indigenous peoples; and an initiative to protect access to care for trans and non-binary youth through addressing disinformation and misinformation.
Modernizing the interpretation of (and strengthening compliance with) the CHA
The Department will monitor changes in the delivery of health care to ensure that insured services under the CHA remain covered regardless of how the care is provided or who is providing the care.
Health Canada will also work with P/Ts to encourage compliance with the Department’s Diagnostic Services Policy. Jurisdictions will have the opportunity to be reimbursed for any deductions to their Canada Health Transfer if they eliminate patient charges, and the circumstances that led to them, within 2 years of these deductions. This is in keeping with Health Canada’s April 2020 Diagnostic Services Policy for P/Ts, which required P/Ts to report on patient charges for diagnostic services for the first time in December 2022.
Combatting cancer
In 2023-24, Health Canada will invest $47.5 million in the Canadian Partnership Against Cancer to continue improving cancer control in Canada. Grounded in the 2019-2029 Canadian Strategy for Cancer Control, the Partnership will: coordinate and accelerate the adoption of new knowledge and approaches that advance cancer control; focus on issues from prevention and early diagnosis through to survivorship and end of life care; and build on its commitment to develop a more accessible and equitable cancer system for all people in Canada.
The Department will also provide ongoing support for ovarian cancer research by providing $2.25 million in funding to Ovarian Cancer Canada to address gaps in knowledge about effective prevention, screening, and treatment options.
Health Canada will continue to support the expansion of a national network of cancer centres to advance precision medicine in cancer research by providing over $35.6 million to the Terry Fox Research Institute.
Supporting organ, tissues and blood donation and transplantation
The Department will provide funding in 2023-24 of $17.8 million to support the development of clinical practices and public education materials as they relate to organ and tissue donation and transplantation (i.e., clinical tools and resources to support health care professionals, peer-reviewed articles). This funding will also support the development of a national strategy for pediatric and neonatal donation and transplantation, and the development of an education portal for youth, students and teachers, among others. Health Canada will also support research to both improve the safety and supply of the Canadian blood system (i.e., research in transfusion medicine and bone marrow transplant) as well as inform safe and non-discriminatory blood and plasma donation policies. Further, it will also support the construction and start-up of dedicated plasma collection sites across the country to increase domestic sufficiency.
Health Canada will work with stakeholders to improve organ donation and transplantation through the Organ Donation and Transplantation Collaborative. Priorities for 2023-24 include supporting a pan-Canadian governance framework and a pan-Canadian data and performance reporting system.
Improving equitable access to care
Budget 2021 provided Health Canada with $14.9 million in funding to address anti-Indigenous racism in health care. This funding was used to establish Health Canada’s new Addressing Racism and Discrimination in Canada’s Health Systems Program, which provides contribution funding for projects that address systemic racism and discrimination in Canada’s health systems in a way that is informed by the lived and living experiences of Indigenous, racialized, and marginalized communities. The Program’s inaugural open Call for Proposals that closed on May 25, 2022, targeted proposals addressing anti-Indigenous racism, with priority given to projects that aim to develop and implement cultural safety training and/or accreditation requirements, as well as projects that aim to integrate culturally-safe care into acute care settings. In 2023-24, Health Canada will deliver the second year of funding to successful applicants. The Program is also supporting capacity development for Indigenous organizations to engage on their health priorities.
In 2023-24, Health Canada will support projects that address systemic racism and discrimination against Indigenous peoples in Canada.
Did you know?
In 2023-24, Health Canada will contribute $9.3 million to projects that address anti-Indigenous racism and discrimination in Canada’s health systems. This includes support for activities such as developing anti-racism and discrimination training, tools and resources for health professionals, and developing standards and guidelines for cultural safety. The Department will also provide $300,000 to Indigenous organizations to enable them to engage on health priorities.
The Department will provide $37.4 million in 2023-24 under the Official Languages Health Program to improve access to health services for English-speaking communities in Quebec and French-speaking communities elsewhere in Canada. Funded initiatives will focus on:
- Enhancing the availability of bilingual health service providers across the country through post-secondary and language training, to better serve these communities;
- Developing strategies and partnerships with health system stakeholders to improve access to health services for members of official language minorities communities (OLMCs) in Canada through community networking;
- Supporting innovative projects to improve access to health services for OLMCs, in their official language of choice.
Health Canada will continue to meet the lifetime needs of Canadian thalidomide survivors through the Canadian Thalidomide Survivors Support Program, allowing them to age with dignity. In 2023-24 the Department will provide over $11 million in funding to eligible survivors and an additional $1.1 million in funding through the Extraordinary Medical Assistance Fund to help pay for specialized surgeries, home and vehicle adaptations not covered by P/T health care plans, as well as some ongoing costs such as attendant services and physiotherapy. This Program provides a fair and comprehensive approach to identifying thalidomide survivors that is based on international best practices. In 2023-24 the Program will also focus on assessing applications using a newly revised and streamlined process as Health Canada remains committed to identifying opportunities for service delivery improvement.
Key Risk(s) for Core Responsibility 1: Health Care Systems
- Key examples of Health Canada’s planned risk responses
| Monitoring and Reporting on compliance |
|
|---|---|
| Implement new policies | Work with P/Ts to ensure reporting on and adherence to the Diagnostic Services Policy, which clarifies that patient charges for diagnostic services are not permitted under the CHA. |
| Work to resolve issues with P/Ts | Work with P/Ts to resolve CHA issues when deductions are necessary; communicate the process required for a P/T to receive a reimbursement as stipulated in the Reimbursement Policy. |
| Monitor litigation | Monitor any litigation that may implicate the CHA, and support federal involvement as required. |
| Monitor changes in health care delivery |
|
- Risk: Health Canada’s ability to achieve its mandate may be at risk due to challenges posed by major disruptive events such as climate change and pandemics.
| Provide timely, trusted and evidence-based information |
|
|---|---|
| Facilitate access to health products | Support the prevention and treatment of novel diseases via clinical trials and flexible measures. For example:
|
| Foster engagement and collaboration | Continue to work alongside other government departments (e.g., PHAC, PSPC) to advance a whole-of-government approach to adapt to and meet the needs of Canadians. For example:
|
| Enhance internal services | Continue to deliver services and commitments during major crises. For example:
|
Planned results for Core Responsibility 1: Health Care Systems
The following table shows, for Core Responsibility 1: Health Care Systems, the planned results, the result indicators, the targets and the target dates for 2023–24, and the actual results for the three most recent fiscal years for which actual results are available.
| Departmental Result Indicators | Target | Date to achieve target | Actual Results |
|---|---|---|---|
| National health expenditure as a percentage of Gross Domestic Product (GDP) (Baseline: 11.0% of GDP in 2014-15) |
Between 10.9% and 13.4%Table 1 note 1 | March 31, 2024 | 2019-20: 11.7% 2020-21: 13.8% 2021-22: 13.2% |
| Real per capita health expenditure (1997)Table 1 note 2 (Baseline: $4,074 per person in 2014-15) |
Between $4,386 and $5,361Table 1 note 1 | March 31, 2024 | 2019-20: $4,421 2020-21: $4,759 2021-22: $4,963 |
| DrugTable 1 note 3 spending as a percentage of Gross Domestic Product (Baseline: 1.7% in 2014-15) |
Between 1% and 2% | March 31, 2024 | 2018-19: 1.7% 2019-20: 1.8% 2020-21: 1.9% |
| Percentage of family physicians using electronic medical records (Baseline: 73% in 2015) |
At least 95% | March 31, 2026 | 2019-20: 86% 2020-21: 86% 2021-22: 86%Table 1 note 4 |
Table 1 Notes
|
|||
| Departmental Result Indicators | Target | Date to achieve target | Actual Results |
|---|---|---|---|
| Percentage of Canadians (aged 15+) with a mental disorder who have expressed that they have an unmet mental health care need (Baseline: 26% in 2012) |
At most 22% | March 31, 2027 | 2019-20: 24.8% 2020-21: 24.7%Table 2 note 1 2021-22: 24.7%Table 2 note 2 |
| Percentage of Canadians (aged 18+) who have expressed that they have an unmet need for access to home care services (Baseline: 1.6% in 2015-16) |
At most 1% | March 31, 2027 | 2019-20: 1.7% 2020-21: 1.3%Table 2 note 1 2021-22: 1.7% |
| Percentage of Canada Health Act compliance issues addressed within 24 months of identification (Baseline: 80% in 2019-20)Table 2 note 3 |
At least 80%Table 2 note 4 | March 31, 2024 | 2019-20: 80% 2020-21: 83% 2021-22: 80% |
| Percentage of Canadians (aged 12+) who did not fill a prescription for medicine or skipped doses of medicine because of the cost (Baseline: 7.1% in 2014) |
At most 5% | March 31, 2025 | 2019-20: 5.0% 2020-21: 5.0% 2021-22: N/ATable 2 note 5 |
Table 2 Notes
|
|||
Financial, human resources and performance information for Health Canada’s Program Inventory is available on GC InfoBase.
Planned budgetary financial resources (dollars) for Core Responsibility 1: Health Care Systems
The following table shows, for Core Responsibility 1: Health Care Systems, budgetary spending for 2023–24, as well as planned spending for that year and for each of the next two fiscal years.
| 2023-24 budgetary spending (as indicated in Main Estimates) |
2023-24 Planned spending |
2024-25 Planned spending |
2025-26 Planned spending |
|---|---|---|---|
| 2,958,177,598 | 2,958,177,598 | 2,730,077,364 | 2,184,574,828 |
|
Note: The decrease in planned spending in 2024-25 is mainly due to funding level decreases to implement the interim Canada Dental Benefit Plan; as well as the expiry of budgetary spending authorities for mental health supports and services; the Canadian Drug Agency Transition Office; and supporting access to sexual and reproductive health care information and services.
The decrease in planned spending in 2025-26 is mainly due to the expiry of budgetary spending authorities to develop a national strategy for high-cost drugs for rare diseases; and to implement the interim Canada Dental Benefit Plan.
The Department would have to request funding for these initiatives for future years. |
|||
Planned Human resources (full-time equivalents) for Core Responsibility 1: Health Care Systems
The following table shows, in full-time equivalents, the human resources the department will need to fulfill this core responsibility for 2023–24 and for each of the next two fiscal years.
| 2023-24 Planned full-time equivalents |
2024-25 Planned full-time equivalents |
2025-26 Planned full-time equivalents |
|---|---|---|
| 385 | 299 | 272 |
|
Note: The decrease in planned FTEs in 2024-25 is mainly due to funding level decreases to implement the interim Canada Dental Benefit Plan; as well as the expiry of budgetary authorities for the Canadian Drug Agency Transition Office; and mental health supports and services.
The decrease in planned FTEs in 2025-26 is mainly due to the expiry of budgetary spending authorities to implement the interim Canada Dental Benefit Plan; and to develop a national strategy for high-cost drugs for rare diseases.
The Department would have to request funding for these initiatives for future years. |
||
Financial, human resources and performance information for Health Canada’s Program Inventory is available on GC InfoBase.
Core Responsibility 2: Health Protection and Promotion
Description
Health Canada works with domestic and international partners to assess, manage, and communicate the health and safety risks and benefits associated with health and consumer products, food, chemicals, pesticides, environmental factors, tobacco and vaping products, cannabis, and controlled substances.
Planning Highlights
The Department will continue to advance the Regulatory Innovation Agenda, a multi-year regulatory modernization plan designed to make the federal regulatory framework more agile and responsive to an innovative environment. This in turn will help companies in the health and biosciences sector bring products to the market while ensuring the system remains science and safety-based.
The Agenda cuts across multiple Departmental Results that make up Core Responsibility 2. Its continued implementation will result in modernized policies, frameworks and regulations for health products and foods that protect the health and safety of Canadians, while encouraging innovation.
Health Canada will continue to build on the lessons learned from the temporary regulatory measures launched in response to the pandemic to inform policy and regulatory development as it continues to implement its commitments described in the Health and Biosciences: Targeted Regulatory Review – Regulatory Roadmap and Agri-food and Aquaculture and Regulatory Review Roadmap.
Departmental Result 3: Canadians have access to safe, effective and quality health products
Over the course of 2023-24, Health Canada will continue efforts to ensure Canadians have timely access to safe, effective and quality health products – including prescription and non-prescription pharmaceutical drugs, biologic and radiopharmaceutical drugs, natural health products, and medical devices – and to support the needs of the healthcare system. Improvements will help accelerate market access for innovative, breakthrough products along with cost effective alternatives, such as generic and biosimilar drugs.
The Department will focus on the following priorities: providing timely access to health products; managing and monitoring drug and medical device availability; applying real-world evidence to support regulatory decision-making; strengthening regulatory oversight; modernizing compliance and enforcement; acting to prevent and control antimicrobial resistance (AMR); fostering international collaboration and coordination; as well as promoting access to new and emerging technologies.
Providing timely access to health products
In 2023-24, the Department will continue to provide Canadians with timely access to health products by reviewing the safety, efficacy and quality of pharmaceutical and biologic drugs, medical devices and natural health products. The Special Access Program allows health care professionals to request, in emergency situations, medical devices and drugs not yet authorized for use in Canada. Health Canada developed an electronic tool (eSAP) to allow these professionals to submit requests electronically, follow up on the status of requests, and receive decisions electronically via their mobile devices. The Department will implement the eSAP platform to provide an end-to-end electronic solution for Health Canada’s special access programs to fully support their regulatory responsibilities and to improve the user experience and service. Health Canada will also work closely with P/Ts, international regulatory partners, industry and health professionals to anticipate and meet the needs of Canadians for health products.
In addition, the Department will advance plans to entrench regulatory agilities piloted during the COVID-19 pandemic into regulatory frameworks. Health Canada is consulting with stakeholders on a proposed expansion of the COVID-19 requirements under the Food and Drug Regulations. Based on lessons learned through the pandemic, the Department is developing provisions in the Regulations to establish a Public Health Emergency Drug pathway that would extend the flexibilities introduced for COVID-19 to new and emerging public health emergencies.
Similarly, the Department is developing amendments to the Medical Devices Regulations to maintain the same authorization mechanisms relating to the importation and sale of medical devices for use in relation to COVID-19 following the expiry of the interim order on February 21, 2023. This will ensure regulatory stability, continued expedited access to COVID-19 medical devices with a public health need, and will allow existing interim order authorizations and expanded use indications to be maintained.
The Department will also continue to prioritize its scientific review of drugs, vaccines and medical devices for urgent public health needs, including those for COVID-19. As part of its continued efforts to be open and transparent, Health Canada will continue to publish high-level summaries of the evidence it has reviewed to support authorizations.
The Department and PHAC are working closely with manufacturers, P/Ts, public health authorities and international regulators to monitor the safety and effectiveness of authorized health products, including for COVID-19, and to rapidly investigate and mitigate any risks. Health Canada will also work with partners to protect Canadians from false and misleading health product advertising, including for COVID-19. Furthermore, the Department will continue to advance policy, regulatory and operational initiatives to modernize the regulatory oversight of generic drugs.
Did you know?
In 2023-24, Health Canada will further develop and implement its SGBA Plus Action Plan as it relates to health products to better integrate SGBA Plus considerations across the health product lifecycle. This includes finalizing the policy work for a regulatory amendment that would require the submission of disaggregated data from clinical trials, as part of the Agile Licensing Framework, with consultations beginning in 2023. (see SGBA Plus section for further details).
As part of the Department’s efforts to support priority populations, Health Canada will continue to implement the Pediatric Drug Action Plan to address challenges in accessing safe and effective health products for children and youth in Canada. A proposed amendment to the Food and Drug Regulations is expected to be piloted in 2023-24, where sponsors will be asked to voluntarily provide pediatric data or a pediatric plan with their submissions. Additionally, the Department plans to develop, in collaboration with external experts from the pediatric medical community, a National Priority List of Pediatric Drugs to improve access to those that address the highest unmet medical needs in Canada. Health Canada aims to publish the list in 2023-24 following a public consultation.
Managing and monitoring drug and medical device availability
What’s new?
In November 2022, Health Canada established an internal Drug Shortages Task Force to support and coordinate the short- and long-term response to growing supply challenges of drugs, medical devices, and health products. The Task Force supported Ministerial engagement with stakeholders on shortages of children’s analgesics, antibiotics and adult cough and cold medicines; increased surveillance and data collection to improve supply and demand analysis for critical shortages; and, began research and analysis to inform medium- to longer-term policy options aimed at supporting a more resilient drug supply for Canada so that Canadians have access to the drugs they need when they need them.
The Department led and mobilized stakeholders to release 3.8 million units of domestically-manufactured analgesics to the market in November and December 2022, with a reliable supply for the coming months, and authorized approximately 2 million units of imported foreign-labelled product, without compromising Canada’s high standards for safety, efficacy and quality.
In partnership with P/T governments, drug manufacturers, distributors, wholesalers, and retailers, and patient and health care groups, Health Canada will continue to play a leadership role in mobilizing efforts to alleviate the impacts of drug shortages on Canadians and to try to prevent them, where possible. In 2023-24, the Department will work with stakeholders to strengthen its approach in managing shortages. This will include:
- Broadening stakeholder engagement to better understand the impact of shortages on at-risk populations such as children;
- Improving signal detection to make prevention more feasible more often;
- Increasing the use of data and analytics to help map out more reliable supply and demand information to inform prevention and mitigation measures;
- Proposing and consulting on medium- to longer-term policy options aimed at informing the development of new tools and practices to bolster Canada’s health product resilience.
Health Canada will continue to develop amendments to the Food and Drug Regulations to explore how using trusted foreign regulators’ approvals would support the authorization of drugs currently not available on the Canadian market. It will also enable animal owners and food producers to access veterinary drugs not presently authorized in Canada for minor use or minor species.
Health Canada will continue to allow for flexibility in early phase clinical trials for cannabis and psilocybin to generate high-quality evidence that can be used to better understand the health risks and benefits of their use for therapeutic purposes. An increase in the availability of authorized drugs provides Canadians with a greater selection of therapeutic options to meet their health needs. High-quality research also helps guide practitioners in making evidence-based decisions when prescribing drugs to patients.
Applying real-world evidence (RWE) to support regulatory decision-making
The Department will continue to apply RWE in support of regulatory decisions to improve the post-market oversight of prescription drugs in Canada, particularly those that treat rare diseases, as well as to inform decision-making for COVID-19 drugs and vaccines. It will maintain collaboration with domestic partners through a Steering Committee, co-chaired by Health Canada and CADTH, to advance learning and develop guidance. Health Canada will also work with CADTH to further align RWE use across the drug life cycle and to improve the accessibility, affordability, flexibility and appropriate use of drugs in Canada.
Strengthening regulatory oversight
Health Canada remains committed to modernizing its approach towards regulating therapeutic products by strengthening the continuous monitoring, assessment, and communication of risks and benefits for drugs and medical devices.
In 2023-24, the Department will advance its regulatory modernization plans, including facilitating a modernized Self-Care Framework that encompasses a risk-based approach to regulatory oversight for all self-care products. Furthermore, to help remove barriers to getting non-prescription drugs on the market, Health Canada will continue to implement policy and operational measures through the Non-prescription Drug Action Plan to provide flexibilities and remove administrative burden for industry while ensuring the safety of Canadians is maintained.
In addition, following the publication of the Agile Licensing for Drugs and Medical Devices proposal, the Department will leverage existing policies and practices and build upon the experiences gained throughout the COVID-19 pandemic. This proposal will help enhance safety while supporting innovation and economic growth. These proposed amendments will facilitate greater oversight to compel drug and medical device licence holders to take the necessary steps to address safety, effectiveness, risks and benefits related to drugs and medical devices at any point in their lifecycles. This will also enhance Health Canada’s ability to have continued oversight, assessment, and communication at both the licensing stage and once the licensed products are on the market. These amendments will empower the Department to more rapidly identify risks and mitigation strategies and ultimately to allow products to remain on the market without disruption. Further, the proposal will support the reduction of regulatory roadblocks to innovation by making Canada’s science-based regulatory system more agile and internationally aligned.
Health Canada will continue to respond to the 2021 recommendations for the Natural Health Product program from the Commissioner of the Environment and Sustainable Development. This follows the publication of the Regulations Amending the Natural Health Products Regulations in 2022. As part of the continued response, Health Canada is seeking additional powers such as the ability to require a recall, charge fees to support regulatory activities and to level the playing field for product lines of similar risk. The Department will also propose amendments to modernize the Natural Health Products Regulations, including through the creation of a risk-based categorization structure and enhanced quality review of products seeking authorization to be available in Canada. These amendments would introduce an annual notification to identify marketed products, revisions to strengthen the requirements for Good Manufacturing Practices, and a risk-based site licensing program.
In addition, the Department will advance work on a single regulatory framework for biocides (i.e., surface disinfectants and surface sanitizers) that aims to provide more flexibility in tailoring application and regulatory requirements for these products and better alignment with international requirements. The proposed regulations would also reduce the regulatory burden on industry while maintaining Health Canada’s oversight so that the Department can take action if any health and safety risks are identified.
Also, as part of its Regulatory Innovation Agenda, Health Canada will continue to modernize the regulations for clinical trials through the development and implementation of a single framework for drugs, medical devices, Natural Health Products and Foods for Special Dietary Purposes. Proposed amendments include enhancing a risk-based approach to the oversight of trials, as well as the ability to add terms and conditions on trial authorizations. The value and feasibility of these elements were validated through experience with the COVID-19 interim orders. The new framework would afford greater flexibility in the safe development of innovative therapies, streamline processes, and align with international best practices regarding oversight and public access to information. Additionally, the Department is advancing a plan to increase the transparency of clinical trials in Canada by requiring the registration of trial information and results in a publicly accessible registry.
In 2023-24, Health Canada will continue to advance proposed changes to the Food and Drug Regulations and the Medical Devices Regulations to support a more agile, risk-based approach to compliance and enforcement oversight for drugs and medical devices, while also reducing the regulatory and operational burden. These amendments would address outdated references in the Food and Drug Regulations related to designated foreign regulatory authorities, and introduce exemptions for packagers, labellers, importers or distributors related to finished product testing for certain drugs. The effectiveness of medical device and drug recalls frameworks in Canada would also be improved through modernizing the reporting requirements for firm-initiated recalls and implementing new reporting requirements for Ministerial-mandated recalls of therapeutic products.
In addition, Health Canada will continue to improve scientific advice for medical device sponsors, increase stakeholder engagement, and clarify adverse reaction reporting requirements for regulated stakeholders such as hospitals. The Department will also continue to work with hospitals to increase awareness and compliance with the hospital mandatory reporting regulations.
Health Canada will continue to advance proposals that improve the efficiency and predictability of regulatory decision-making, while strengthening resilience of the supply chain at the same time. It will do so in a phased approach, beginning with updating existing recall requirements for drugs and medical devices, followed by introducing risk-based approaches to annual licence reviews.
Health Canada will analyze data on health product imports declared electronically in the Canada Border Services Agency (CBSA) Single Window Initiative declaration system to identify import trends and potential shipments of concern. The Department will further explore a modified referral process with the CBSA to reduce the potential of backlogs, with a focus on shipments of highest risk.
Health Canada will continue developing a risk-based Good Manufacturing Practices inspection program for Natural Health Products to support the Department’s increased proactive regulatory oversight of this industry. This work responds to a recommendation from the Commissioner of the Environment and Sustainable Development audit that the Department should develop a risk-based monitoring and inspection program.
Modernizing compliance and enforcement
As part of its Compliance and Enforcement (C&E) Modernization and Transformation priority, in 2023-24, Health Canada will continue to implement modern, agile and innovative C&E approaches for health products by focusing on:
- Adopting virtual and remote tools for inspections where appropriate and working with international partners to explore how these might also improve oversight in foreign inspection programs;
- Improving data, analytics and risk management tools to inform decision-making;
- Strengthening internal inspector designation policies, directives, procedures and certification standards;
- Ensuring that inspectors have the necessary tools to exercise their powers and perform their duties in a safe manner through occupational health and safety practices;
- Leveraging technology to ensure C&E designation and training is accessible, agile and consistent;
- Developing a standardized procedures-manual and training for investigations and strategic litigation support.
Acting to prevent and control antimicrobial resistance (AMR)
AMR continues to be an urgent issue for the health of humans, animals and their shared environment. Several initiatives are underway to facilitate access to new antimicrobial drugs for human use and to preserve the effectiveness of existing antimicrobials for use in humans and animals. Key initiatives for 2023-24 include:
- Encouraging manufacturers to submit for review innovative antimicrobials for human use;
- Continuing efforts to raise awareness on the growing threat of AMR, including creating a Canadian list of reserve antimicrobials and developing and distributing educational material to patients on the appropriate use of antimicrobials;
- Updating product labels of select veterinary antimicrobials which do not have clearly defined durations of use, so that these drugs are used responsibly, do not compromise public health, and remain effective for future generations;
- Publishing annual sales data on medically important antimicrobials intended for use in animals to support surveillance stewardship actions;
- Continuing to facilitate access to low-risk veterinary health products that improve health and wellness in animals so that there is less need for routine use of antimicrobials;
- Prioritizing the review of applications for rapid testing devices that can distinguish between types of infections and/or antibiotic resistant genes;
- Establishing a new research program to better understand antibiotic resistance and developing tools to support the development of antibiotic alternatives;
- Continuing engagement with international partners, including the International Coalition of Medicines Regulatory Authorities, the Transatlantic Task Force on Antimicrobial Resistance and other partnerships across human and animal health product sectors to exchange information and inform best practices.
Fostering international collaboration and coordination
Health Canada’s commitment to international partnerships will continue in 2023-24, including supporting access to drugs via bilateral arrangements with foreign regulators and international multilateral initiatives/networks, and advancing initiatives such as work-sharing, as well as:
- Sharing work, intelligence, enhanced confidentiality arrangements, joint review and registration processes (e.g., for human and veterinary drugs, Access Consortium, innovative initiatives such as the European Medicines Agency OPEN initiative on COVID-19 products and the United States Food and Drug Administration’s (FDA) Project Orbis for oncology drugs);
- Developing joint standards (e.g., through Harmonization of Good Manufacturing and Good Pharmacovigilance Practices, and by supporting the work of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use);
- Participating in pilot projects (e.g., eSTAR and Medical Device Single Review Program) to improve patient access to medical devices and support innovation;
- Standardizing data (e.g., clinical trial patient-reported outcomes);
- Harmonizing risk management plans and surveillance (Good Pharmacovigilance Practices);
- Participating in Good Clinical Practices and Bioequivalence clinical trials (to share best practices and deliver international symposiums);
- Continuing to strengthen the joint/simultaneous review processes for veterinary drugs;
- Continuing implementation of additional guidelines from the International Cooperation on Harmonization of Technical Requirements for Registration of Veterinary Medicinal Products;
- Building on existing practices to further leverage the use of foreign reviews, international collaboration, priority review, and proportional levels of review effort based on risk, the Department will explore legislative/regulatory options to designate health products for the purposes of prioritizing access by Canadians or managing risks.
Did you know?
In 2022 the Access Consortium (Australia-Canada-Singapore-Switzerland-U.K.) completed a work-share to authorize a treatment for macular degeneration and another for the treatment of Philadelphia chromosome-positive chronic myeloid leukemia. This first-time collaboration involving all five agencies provided a combined population of 150 million people with access to two medicines.
These five agencies will continue to focus on work-sharing as per their 2021-24 Strategic Plan, while also exploring ways to maximize collaboration, including post-market activities and regulatory innovation.
Collaborating with international partners and continuing to coordinate inspection efforts is important as it allows Health Canada to strengthen compliance and enforcement oversight and information-sharing, and avoid duplication of efforts in order to focus on high-risk priorities.
Promoting access to new and emerging technologies
Scientific and technological advances are accelerating the pace of innovation in the healthcare system, leading to the development of innovative health products that use technologies such as advanced artificial intelligence (AI) and machine learning (ML) algorithms, telerobotics, 3D printing and gene editing. Increasingly, health products are becoming personalized, developed at point of care, and manufactured, distributed, and used in significantly new and non-traditional ways.
Did you know?
Health Canada will continue to advise the work of the Setting International Standards in Analyzing Patient-Reported Outcomes and Quality of Life Endpoints in Cancer Clinical Trials – Innovative Medicines Initiative project.
In 2023-24, the project will analyze patient feedback about their well-being and functioning while on new cancer therapies, develop additional recommendations to improve how to incorporate patient feedback and generalizing some of the findings to other therapeutic areas.
To keep pace with these advances, Health Canada will establish a new gene therapy regulatory research laboratory, building capacity and expertise to assess these new technologies.
In addition, the Department will:
- Publish guidance on pre-market considerations for AI/ML in medical devices;
- Continue to consult its Scientific Advisory Committee in Digital Health Technologies;
- Co-chair the International Medical Device Regulators Forum (IMDRF) Software as a Medical Device Working Group;
- Participate in multilateral Digital Health collaborations with the U.S. FDA, the U.K. MHRA, the European Commission, Denmark, Germany, Australia and the IMDRF AI Working Group.
Health Canada will also explore the use of AI in pharmacovigilance with the goal of eventually developing guidance documents for industry and harmonizing expectations with those of other regulators.
The Department will further implement its new regulatory framework to accommodate Advanced Therapeutic Products (ATP), drugs or devices that are so complex or distinct that they significantly challenge our current regulatory system. As a key component of Health Canada’s Regulatory Innovation Agenda, this work will support timely access for patients while optimizing safety and benefits, as well as strengthening innovation in the health and biosciences sector. Tailored regulatory requirements are being developed for products. The first two ATP pathways currently being explored are:
- Fecal Microbiota Therapy – the transfer of bacteria into a patient’s intestinal tract to establish a healthy microbial community to help fight antimicrobial resistance, such as when treating recurrent C. difficile infections;
- Adaptive Machine Learning-Enabled Medical Devices – medical devices using adaptive algorithms that learn from new datasets and are intended to be updated frequently to improve performance.
Over the course of 2023-24, Health Canada intends to consult on proposed requirements for Adaptive Machine Learning-Enabled Medical Devices.
Departmental Result 4: Canadians are protected from unsafe consumer and commercial products and substances
Helping Canadians lead healthier lives and providing protection from unsafe consumer and commercial products and substances remains a vital component of Health Canada’s work. Over the course of 2023-24, the Department’s efforts in this regard will focus on the following priorities, detailed further below: applying a comprehensive approach to substance use-related harms; regulating cannabis; managing the health risks of chemicals in the home, the workplace and the environment; supporting the safety of consumer products and cosmetics; protecting Canadians from radiation; as well as strengthening pesticide regulation and transparency.
Applying a comprehensive approach to substance use-related harms
Substance use-related harms continue to cause devastating health and social effects on Canadians from every walk of life. The overdose crisis in particular continues to have significant impacts on Canadian communities and families. For example, between January 2016 and June 2022, coroners’ reports recorded 32,632 lives lost to apparent opioid toxicity, with a disproportionate impact on men, on individuals between the ages of 20-59 and on Indigenous Peoples. The COVID-19 pandemic has worsened long-standing challenges regarding substance use and the overdose crisis. From January to June 2022, approximately 20 Canadians were dying each day as result of opioid toxicity as compared to 12 per day in 2018. From January to June 2022, just under half (47%) of accidental apparent opioid toxicity deaths also involved a stimulant, reflecting the polysubstance nature of this crisis. People who use drugs also face additional barriers and risks, such as the highly toxic and unpredictable illegal drug supply, overburdened health and social services, and ongoing stigma surrounding substance use that discourages people from seeking health and social services, and can reduce the quality and availability of services received.
The Canadian Drugs and Substances Strategy outlines the GOC’s approach to addressing substance use with the goal of minimizing harms to individuals, families and communities. It addresses a broad range of substances, including cannabis, tobacco, illegal drugs and alcohol. In 2023-24, the Department will continue work on updating the Strategy to reflect expert advice, lessons and feedback from public consultations on drug policy undertaken in 2018, and evidence gathered from the impact of the pandemic, Ministerial outreach to stakeholders in winter 2022, the Ministerial Expert Task Force on Substance Use, the Canadian Pain Task Force and other significant inputs to strengthen the GOC’s approach to substance-related harms. This engagement has informed GOC actions on substance use and the overdose crisis, such as priorities for the Substance Use and Addictions Program, public education and awareness campaigns, and supporting access to harm reduction and treatment.
In 2023-24, Health Canada will continue working with all levels of government, partners, stakeholders and people with lived and living experience to take a comprehensive approach in addressing substance use and the overdose crisis, including monitoring national trends in the use of alcohol, psychoactive pharmaceuticals and illegal drugs. This will involve general population surveys and implementing a wide range of evidence-based measures to help save lives and minimize substance-related harms for individuals, families and their communities. The Department will also continue to support greater access to services offering safer, pharmaceutical-grade alternatives to the highly toxic illegal drug supply, with the goal of saving lives and reducing drug overdoses. This includes support for effective models and pilot projects, working to build the evidence base around safer supply, and ongoing engagement with key stakeholders. Health Canada will facilitate access to harm reduction services like supervised consumption sites and safer supply services, and support policies and approaches that divert people who use drugs away from the criminal justice system and toward appropriate health service and social supports.
Did you know?
Unmanaged chronic pain and the trauma and complexity that often accompany it is a primary risk factor for substance use. Improving pain management can help reduce first exposure to opioids and prevent harms associated with substance use. Health Canada will continue to coordinate federal efforts to share best practices to address concurrent chronic pain and substance use related conditions.
Over the coming fiscal year, the Department will continue to work with domestic and international partners to improve access to treatment services for substance use disorder. Addressing stigma, people impacted by social marginalization and taking a public health approach to drug policy will continue to underline Canada’s engagement internationally. Recognizing that substance use can increase the underlying risk of mental health issues and exacerbate existing issues, the Department will also explore integrated approaches to treating mental health, chronic pain, and other disorders, as well as evidence-based interventions to reduce stigma.
In 2023-24, Health Canada will continue to invest in public education, raising awareness of substance use harms and the importance of reducing the stigma towards people who use drugs. The Department will further continue to reach higher-risk populations – including children and youth, and men 20-60 years of age working in trades, Indigenous Peoples, Black Canadians and other racialized groups – to reduce the stigma associated with seeking help. Additionally, Health Canada plans to engage healthcare system leaders to raise awareness of the issue of structural stigma and mobilize action to address it.
In response to the devastating effects of the overdose crisis and broader substance use, Health Canada will continue to support organizations at the community, regional and national levels through its Substance Use and Addictions Program, including providing approximately $10 million per year to the Canadian Centre on Substance Use and Addiction to address substance use at a coordinated, national level. Other priorities include:
- Improving access to prevention, harm reduction and treatment services, naloxone training and distribution, and the safer supply of prescription opioids;
- Furthering public education and awareness about the use of tobacco, vaping and alcohol products;
- Strengthening the capacity among the substance use workforce to promote consistent service delivery and promoting an integrated and coordinated treatment system.
Health Canada will continue to provide analytical services and intelligence on illegal controlled substances for public health purposes. This includes delivering timely and reliable information such as Drug Notifications, Drug Summary Reports, and Drug Factsheets to Canadian law enforcement agencies, public health partners and the public. The Department will strengthen its collaboration with national partners and agencies to support harm reduction initiatives such as checking drug samples from supervised consumption sites for toxic substances like fentanyl, and promoting, monitoring verifying and enforcing compliance with the Controlled Drugs and Substances Act and its Regulations.
Regulating cannabis
The purpose of the Cannabis Act is to protect public health and public safety – in particular, the health of young persons by restricting their access to cannabis – while providing adults with legal access to regulated products and reducing illegal activities involving cannabis.
In 2023-24, Health Canada will support effective implementation of the cannabis legislative and regulatory framework in collaboration with P/Ts, Indigenous communities, the regulated industry, public health organizations, academics, federal partners and law enforcement. The Department will issue licences and permits under the Act, including for cannabis, hemp, research, analytical testing and for drugs containing cannabis, facilitate reasonable access to cannabis for medical purposes, as well as conduct compliance and enforcement activities.
The Department will develop regulatory amendments to simplify and streamline administrative requirements and will continue to provide guidance and interpretation of cannabis-related legislative and regulatory requirements. In line with the Cannabis Fees Order, Health Canada will continue to administer the cost recovery framework, and in keeping with the Cannabis Tracking System Order, the Department will continue to manage the national Cannabis Tracking System, which provides information on cannabis production, inventories, distribution and sales.
Health Canada will continue to promote a diverse commercial cannabis industry and deter illicit activity by reducing barriers, improving guidance, and enhancing outreach for micro-cultivation and micro-processing licence applicants who wish to grow or process cannabis on a smaller scale. The Department will continue to offer an Indigenous Navigator Service for Indigenous-affiliated parties who are interested in obtaining a federal licence to cultivate or process cannabis, including industrial hemp, under the Act.
What’s new?
In 2023-24, an independent Expert Panel appointed to undertake a legislative review of the Cannabis Act will engage directly with the public, stakeholders and Indigenous partners and provide advice to the Minister of Health and the Minister of Mental Health and Addictions and the Associate Minister of Health on progress made towards achieving the Act’s objectives. The Panel will also provide the Ministers with findings or recommendations to strengthen the administration and operation of the Cannabis Act.
Health Canada will also continue providing reasonable access to cannabis for medical purposes by registering individuals who have the support of their health care practitioner to produce a limited amount of cannabis for medical purposes or to designate someone to produce it for them. The Department will strengthen the integrity of the medical access framework by addressing the potential public health and public safety risks posed by individuals who may misuse the personal production of cannabis for medical purposes. For example, Health Canada will request additional evidence from health care practitioners to substantiate or support high daily authorization amounts. The Department will also promote information on the regulations around cannabis for medical purposes among the public as well as information on dosing with health care professionals.
Health Canada will further promote, monitor, verify and enforce compliance with legislative and regulatory requirements. The Department will review and act upon incidents of potential non-compliance and will develop broad, reactive strategies to address observed patterns of non-compliance. Additionally, Health Canada will continue to review notices of new cannabis products before these enter the Canadian marketplace by assessing, investigating, and responding to compliance-related questions and complaints; as well as reviewing, assessing and responding to observations arising from federal inspections of licence holders’ facilities.
What’s new?
Health Canada’s Science Advisory Committee on Health Products Containing Cannabis concluded that there is early evidence to suggest that cannabidiol is:
- Effective for the short-term treatment of mild symptoms associated with stress and nervousness;
- Safe in doses up to 200 mg/day taken orally for short-term use by healthy adults;
- Not habit forming.
Health Canada’s Science Advisory Committee on Health Products Containing Cannabis published its final report in July 2022. The report focused on cannabidiol (CBD) and provided advice on the appropriate safety, efficacy and quality standards for health products containing CBDs for both human and animal use, including the conditions under which these products would be suitable for use without practitioner oversight. This advice will help inform a potential approach to permit the use of CBD in certain low-risk products that would not require a prescription for people and animals. With the release of the final report, Health Canada issued a Notice to Stakeholders to gather feedback and evidence from potential consumers and stakeholders to help address outstanding knowledge gaps, including drug and food interactions, long-term safety, possible effects on pregnancy, information on at-risk populations, species-specific evidence for use in animals, and human safety implications for use in food-producing animals. In 2023-24, Health Canada will analyze the feedback received from stakeholders as the Department continues the policy development process for a potential framework for health products containing CBD.
The Department will also continue to closely monitor the public health and safety impacts of the Cannabis Act. Health Canada monitors, collects and analyzes scientific evidence and data on cannabis and conducts research and surveillance on cannabis; and monitors, assesses and publicly reports on adverse reactions and communicates potential health risks of cannabis use to Canadians through public reports. In 2023-24, the Department will continue to publish public health advisories in response to reports of risks, including accidental ingestion of cannabis.
Managing the health risks of chemicals in the home, the workplace and the environment
Health Canada will continue to identify and manage the risks of chemical substances to protect the health of Canadians. As part of its ongoing commitment to chemicals management, the Department will conduct further research, monitoring and surveillance (including biomonitoring) and risk assessments regarding new and existing chemical substances and products of biotechnology, and will take appropriate action to help protect the health of Canadians. This work will be supported by international collaboration to leverage the best available expertise and information. Additionally, Health Canada will continue to lead Canada’s engagement in the development of a robust voluntary global framework for the sound management of chemicals and waste. This framework is expected to be finalized and adopted in September 2023. Through the Healthy Home Campaign, the Department will also continue to develop and use interactive and digital tools to inform the public, including disproportionally impacted Canadians, about the potential risks from harmful chemicals and the actions they can take in and around their homes to protect their health.
Did you know?
The GOC has a comprehensive plan to achieve zero plastic waste by 2030, prevent plastic pollution and transition to a circular plastics economy, in which plastic never becomes waste. Instead, plastics are reused, recycled, or their use is eliminated or replaced with more sustainable materials. Moving toward a more circular economy for plastics could reduce carbon emissions by 1.8 megatons annually, generate billions of dollars in revenue, and create approximately 42,000 jobs by 2030.
Health Canada will continue to conduct research and will invest in Canadian academic research to better understand the potential effects of microplastics on human health and to support the GOC’s agenda for reducing plastic waste. The Department will develop methods to better assess microplastics in food, begin studies to support the reassessment of titanium dioxide (TiO2), and, as part of the Canadian Total Diet Study, continue to monitor chemical contaminants transferred from food. Further, Health Canada will continue to collaborate with Environment and Climate Change Canada and other departments to advance a circular plastics economy for Canada.
The Department will also continue to work with Environment and Climate Change Canada to strengthen the Canadian Environmental Protection Act, 1999.
For the first time, the Canadian Health Measures Survey, currently in its seventh cycle, will collect data on exposures to environmental chemicals in children aged 1-2 years. This inclusion will provide key information about early life exposures to environmental chemicals in Canadians. Results are expected in fall 2026.
To mitigate risks posed by workplace hazardous products, Health Canada will continue to conduct hazard assessments, collaborate on the enforcement of appropriate labelling and communication of hazards and undertake outreach activities to increase awareness of the Hazardous Products Act and Hazardous Products Regulations. These efforts will be informed and supported by international cooperation, including through implementation of revised editions of the Globally Harmonized System of Classification and Labelling of Chemicals and participation in the United Nations Sub-Committee of Experts on the same, and in the Canada-U.S. Regulatory Cooperation Council.
Health Canada will also continue to develop and modernize policies and operational procedures pertaining to workplace hazardous products under the Hazardous Products Act and the Hazardous Materials Information Review Act and their Regulations. For example, the Department will address the exclusion of consumer products from workplace labelling and safety requirements and leverage efficiencies in the review of applications from industry seeking to protect confidential business information under the Hazardous Materials Information Review Act.
Health Canada will continue to investigate food contaminants such as mercury and bisphenols using the Canadian Total Diet Study and other targeted studies. Working with F/P/T partners, the Department will mitigate health risks of contaminants in food not sold at retail, but which are traditionally consumed by certain groups (e.g., Indigenous hunting/gathering of foods) by issuing consumption advice for groups at risk. Health Canada will further work with industry to ensure best practices are being followed during food processing and continue to update the regulatory maximum levels for chemical contaminants in food. It intends to publish consumer advice and information for Canadians on several additional topics related to food chemical safety, such as educational material for persons with a mustard allergy.
In 2023-24, the Department will continue to engage with the public and stakeholders on amending the Food and Drugs Act to strengthen the environmental risk assessment and risk management of ingredients in drugs. These amendments will require industry to provide environmental data on new drug ingredients at different stages during the drug development process (e.g., when applying for clinical trial application or for market authorization). This will better align with international regulators and create a more streamlined approach for industry.
Health Canada will update its guidelines on the acceptability and use of recycled plastics in food packaging applications and make these publicly available.
Supporting the safety of consumer products and cosmetics
Did you know?
Canadian poison centres manage over 215,000 cases per year, with most involving products or substances regulated by Health Canada. The Department collaborates with poison centres and other partners to implement the Canadian Surveillance System for Poison Information and to produce Pan-Canadian Poison Centre Annual Reports. These reports share poison-related information with the public and highlight the need for ongoing collaboration and action to reduce poisonings in Canada.
The Department will continue to mitigate risks posed by unsafe consumer products and cosmetics by updating policy and operational procedures, as necessary, and conducting risk assessments, compliance and enforcement, and outreach activities. For example, Health Canada will continue to work with international partners to coordinate joint product recalls.
Health Canada will further strengthen legislative and regulatory requirements pertaining to consumer products under the Canada Consumer Product Safety Act (CCPSA) and to cosmetics under the Food and Drugs Act. The Department will continue to work towards introducing new labelling requirements for chemicals in consumer products and certain fragrance allergens in cosmetics and ending cosmetic testing on animals.
Health Canada will examine ways to address enforcement challenges resulting from the growing global marketplace and the increasing prevalence of e-commerce. For example, the Department will identify options to address challenges in the application and enforcement of the CCPSA and its Regulations for consumer products sold online, such as the advancement of guidance for online sellers and other initiatives to support compliance.
Protecting Canadians from radiation
Health Canada will continue to monitor, advise, and report on exposure to radiation that occurs both naturally and from man-made sources under the authority of the Department of Health Act; the Radiation Emitting Devices Act; the Comprehensive Nuclear Test-Ban Treaty Implementation Act; the Emergency Management Act; and the Nuclear Safety and Control Act and its Regulations. As part of the Federal Nuclear Emergency Plan, the Department will participate in nuclear emergency training, drills and exercises and will coordinate with F/P/T and international partners to confirm that emergency preparedness plans are ready for execution in the event of a nuclear emergency. Additional ongoing activities for 2023-24 include:
- Advancing efforts to modernize and enhance the environmental radiation monitoring platform to provide reliable and accessible radiation data to Canadians daily and during a nuclear emergency;
- Continuing, with stakeholder input, to advance efforts to modernize the Radiation Emitting Devices Act and its Regulations;
- Developing relevant information and science-based advice for Canadians and stakeholders on the safety of these devices, particularly as it relates to noise exposure and health.
What’s new?
Health Canada is working to amend the Radiation Emitting Devices Regulations for lasers to better align with the latest internationally recognized product safety standards and by applying the requirements to a broader range of laser technologies. The proposed regulations would require that laser products imported and sold in Canada have adequate warnings of laser hazards, provide information to support safe use, and that lasers incorporate safety features to help reduce the possibility of eye and/or skin injury to Canadians.
Strengthening pesticide regulation and transparency
Health Canada will continue to make timely, science-based decisions that support the safe and sustainable use of effective pesticide products. The Department will also promote, monitor, and enforce compliance with the Pest Control Products Act (PCPA) and its Regulations. Building on the consultations conducted in spring 2022, in 2023-24 the Department, supported by a targeted review of the PCPA, will continue to transform Canada’s pesticide regulatory system to strengthen its oversight and protection of human health and the environment. In consultation with stakeholders and partners, Health Canada will continue to advance key initiatives by: supporting the transparency of processes and decisions; increasing the availability of independent data to better inform pesticide review decision; and modernizing business processes.
Improving transparency to help Canadians better understand how pesticides are regulated in Canada by:
- Developing new science communication products, including new summaries for certain regulatory decisions that explain in plain language how the Department considers information, conducts assessments, and makes decisions;
- Considering options to further strengthen how Health Canada provides access to industry test data, while continuing to ensure proper data protections are in place;
- Considering an early notification on import maximum residue limit applications to support stakeholders in preparing to make comments on the proposed limit once it is published;
- Continuing to improve user experience of the Department’s web pages to better locate and comprehend information to bolster public trust and participation;
- Improving transparency of the pesticide review process by disclosing the name of registrants at the time that the review process is initiated;
- Continuing to publish an annual report that highlights performance and activities over the past year.
Increasing the use of independent data and independent advice for better-informed pesticide review decisions by:
- Supporting the generation of independent, real-world data from other government departments, P/Ts, universities and non-governmental organizations;
- Commissioning custom surveys that will include key information, including regarding pesticide use by workers;
- Continuing development, in collaboration with partners, of a framework that will support the design and implementation of a national program to monitor pesticide levels in Canada’s lakes, rivers, and groundwater;
- Continuing to work with Environment and Climate Change Canada by investing in Canadian academic research to better understand the potential effects of pesticides on wildlife;
- Establishing the foundation for a new data-centric environment, including new digital tools to facilitate the collection, analysis and disclosure of post-market data on water monitoring and pesticide use, to better inform review decisions and enhance program performance;
- Developing, in partnership with stakeholders and partners, a pesticide use framework supporting the systematic gathering of post-market pesticide use information and data.
Enhancing human health and environmental protection through modernized business processes by:
- Continuing to implement various measures to reduce the re-evaluation backlog;
- Continuing to develop and implement a continuous oversight approach to improve Health Canada’s oversight of pesticides, including building a central data repository of key pesticide information, more frequent stakeholder engagement, and establishing a systematic process for scanning emerging scientific literature on pesticides. These process improvements will empower the Department to be more timely in identifying emerging risks and mitigation strategies and better protect Canadians and the environment. These process improvements also include developing a risk-based approach to the oversight of pesticides;
- Continuing to engage and collaborate with international regulatory partners, such as the United States Environmental Protection Agency, the European Food Safety Authority and the Australian Pesticides and Veterinary Medicines Authority, on work-sharing, joint reviews and the development of standards through the Organisation for Economic Co-operation and Development;
- Establishing the foundation for a new cloud-based platform and digital tools, beginning with a new portal for applicants, a simplified application process, and web-friendly pesticide labels.
Departmental Result 5: Canadians make healthy choices
Helping Canadians make healthy choices in their day-to-day lives is a vital part of Health Canada’s Health Protection and Promotion core responsibility. Over the course of 2023-24, the Department will focus on the priorities, detailed further below: promoting healthy eating; modernizing the regulatory oversight of food, including its packaging and labelling; ensuring the safety and nutritional quality of the Canadian food supply; taking action on youth vaping and reducing tobacco use; as well as supporting Canadians in making informed decisions about cannabis use through public education, research and surveillance.
Promoting healthy eating
Over the coming fiscal year, Health Canada will continue to advance its Healthy Eating Strategy, which aims to curb the rising burden of obesity and chronic disease by making healthier choices easier for people in Canada. It will support restrictions on the marketing of unhealthy food and beverages to children, as well as continue to work with Canadian and international experts to monitor and report on the state of such marketing to children in Canada.
The Department will further promote healthy eating through the creation of new resources to help people increase their knowledge and use of Canada’s food guide in their everyday lives. These include:
- Promoting the development of healthy eating food skills among people living in Canada by increasing awareness and use of the Canada food guide’s kitchen, an immersive web experience that connects recipes, videos, and food skills articles to the food guide’s healthy eating recommendations;
- Developing easy-to-use content to support people living in Canada in using Canada’s food guide, including food skills articles and web content;
- Providing intermediaries with resources and tools to support healthy eating and food guide awareness and education;
- Collaborating with stakeholders to further integrate the dietary guidance into policy, programs and resources.
To instill long-lasting healthy eating habits, children and youth will be key target audiences for Health Canada’s educational and awareness efforts. For example, the Department will refine its approach and explore collaborations to test the implementation of draft principles for healthy eating in campus dining halls, following a pilot with the University of British Columbia that led to an increase in sales of foods aligned with Canada’s food guide.
To encourage further reduction in products with the highest sodium content, Health Canada will continue to work with the food-processing sector to achieve the targets set in the “Voluntary Sodium Reduction Targets for Processed Foods, 2020-25.” The Department also intends to consult on draft proposed targets for reducing sodium in foods sold in restaurants and food services, focusing on those foods Canadians consume most often when eating out. These targets will address a gap in the current approach to sodium reduction.
In addition, Health Canada plans to publish baseline data on the trans-fat intake of Canadians, assessing this against the World Health Organization’s recommendations.
What’s new?
To help Canadians more easily identify foods that are high in sodium, sugars or saturated fat, Health Canada will update guidance for industry on the front-of-package nutrition symbol labelling. By January 1, 2026, a front-of-package nutrition symbol (example image below) will be required on prepackaged foods that are high in these nutrients. Avoiding excess consumption of these nutrients can help reduce associated health risks.
Modernizing the regulatory oversight of food, including its packaging and labelling
The way in which food regulations are currently structured under the Food and Drugs Act limits the Department’s ability to respond to advances in science and technology and makes it difficult for industry to bring innovative products to market. In 2023-24, Health Canada will use modern regulatory tools to address these challenges by:
- Developing regulations to restrict advertising to children of certain foods;
- Modernizing the oversight of microbiological criteria and food additives;
- Engaging with stakeholders to ensure their priorities help inform the Department’s modernization activities;
- Continuing to develop guidance to address emerging food technologies, such as cellular agriculture and gene editing;
- Advancing a regulatory framework that enables clinical trials on foods for a special dietary purpose, such as infant formula or a formulated liquid diet;
- Continuing to consult on and modernize Health Canada’s Lists of Permitted Food Additives;
- Developing an awareness and education strategy to help Canadians interpret and use the new supplemented foods facts table and supplemented foods caution identifier, allowing them to make informed food choices;
- Preparing and planning for nutrition labelling education as a component of healthy eating education;
- Collecting data on changes in the food supply (e.g., sugars, sodium, saturated fats, sweetening agents, claims) to monitor implementation of the front-of-package requirements;
- Finalizing, with the Canadian Food Inspection Agency, voluntary guidance and best practices for nutrition labelling information for foods sold via e-commerce.
Health Canada will collaborate with a variety of stakeholders to both extend the reach of its awareness and education messaging regarding changes to food labels and encourage them to read labels.
Ensuring the safety and nutritional quality of the Canadian food supply
The Department will continue to conduct toxicology research on flame retardant and man-made chemical contaminants in food, with a focus on refining the threshold levels that may pose risks to females versus males, pregnant women and infants.
Canada’s food safety system is one of the best in the world. Health Canada works with other jurisdictions, industry and consumers to establish policies, regulations and standards related to the safety and nutritional quality of all food sold in Canada. In 2023-24, the Department will continue to educate the public and increase awareness about the importance of safe food handling, selection and preparation practices to reduce foodborne illnesses and adverse reactions to food allergens in Canada.
What’s new?
As part of Health Canada’s multifaceted marketing campaign focused on food safety, the Department will target specific populations with a variety of communication and education products under development such as:
The Department will also continue to work with stakeholders to ensure fortified foods are safe and nutritionally adequate, for example by enabling yogurt to be fortified with vitamin D as part of Health Canada’s vitamin D fortification strategy.
Taking action on youth vaping and reducing tobacco use
In 2023-24, Health Canada will continue to address the ongoing high rates of youth vaping and to implement Canada’s Tobacco Strategy – a modernized approach for successful tobacco control with the goal of achieving the target of less than 5% tobacco use by 2035. The Department will continue to support the second review of the Tobacco and Vaping Products Act which will launch in 2023-24 and be tabled in 2024-25. Additional reviews will follow every two years.
In cooperation with other F/T/P partners and key stakeholders, new or ongoing measures to address youth vaping will include:
- Advancing new regulations requiring manufacturers and importers of vaping products to provide information on their products to Health Canada;
- Monitoring national trends in vaping and smoking, particularly among youth and young adults, and conducting public opinion research to better understand youth use of vaping products and inform future regulatory and policy initiatives;
- Inspecting websites where advertising or promotion of vaping products is accessible to youth; inspecting retailers, manufacturers, and importers of vaping products and conducting product sampling and testing; and publishing the results of compliance and enforcement activities;
- Raising public awareness of the potential harms associated with vaping and smoking, particularly for youth, through campaigns such as promoting Consider the Consequences self-/teacher-led virtual sessions;
- Exploring innovations to modernize the Pan-Canadian Quitline Initiative and developing voluntary smoking cessation standards for health care organizations, in collaboration with P/Ts and other stakeholders;
- Providing up to $3.5 million for community organizations that undertake prevention, protection and/or cessation efforts against the use of tobacco and vaping products. Projects will aim to inform Canadians on cessation interventions for people who smoke and for youth who vape, encourage and support attempts to quit, and address any information/knowledge gaps.
What’s new?
Health Canada is taking a person-centered approach to smoking cessation, addressing the stigma surrounding smoking and engaging directly with at-risk populations and people with lived and living experience to ensure policies and programming better reflect the needs of people who smoke. The Department is also considering youth vaping within a broader multi-drug use landscape and its connection to mental health to better understand and address the underlying factors of substance use by young people.
In 2023-24, new or on-going measures related to tobacco will include:
- Advancing new regulations to update the health-related messages (including health warnings) that must be displayed on tobacco products packaging;
- Proposing a framework to require tobacco manufacturers to pay for the cost of federal public health investments in tobacco control;
- Promoting compliance and inspecting tobacco product retailers and manufacturers;
- Communicating with Canadians about new tobacco and/or vaping product regulations as applicable.
Supporting Canadians in making informed decisions about cannabis use through public education, research and surveillance
As the legal cannabis industry increasingly displaces the illegal market and adults of legal age are provided with greater access to regulated cannabis products, Health Canada will continue to closely monitor and evaluate changes in knowledge, attitudes and behaviours (e.g., through the Canadian Cannabis Survey). The Department will adapt its public education and awareness activities, based on emerging evidence, to ensure that Canadians are able to make informed decisions to protect their health and safety.
Evidence-based public education and awareness messaging will focus on: the risks of using cannabis to cope with mental health issues; the impact of cannabis use on the developing brain; and the ongoing risk of pediatric poisonings from cannabis products, including a focus on safe storage and the importance of buying legal cannabis products.
Public education will focus on priority populations (e.g., youth and young adults, pregnant and breastfeeding people, 2SLGBTQI2+ populations, parents, older adults, health professionals, school communities, etc.) and will target higher risk behaviours. Health Canada will continue to regularly engage First Nations, Inuit and Métis leaders, organizations and communities to support a distinctions-based engagement approach, to advance a renewed relationship with Indigenous Peoples, and to respond to specific interests and priority areas raised by Indigenous leadership.
The Department will also continue to develop and implement the new framework for human research on the effects and risks of cannabis for non-medical purposes. This research will in turn generate knowledge to inform public health and safety measures, public education, and policies.
Key Risk(s) for Core Responsibility 2: Health Protection and Promotion
- Risk: Canadians may lose confidence in Health Canada’s ability to help protect their health if the Department is not regarded as a trusted regulator and used as a credible source of information.
| Implement informative initiatives |
Increase and update the regulatory health and safety information that is made available to Canadians in a simple and accessible way. For example:
|
|---|---|
| Offer engagement opportunities to Canadians and stakeholders |
In line with the GOC’s initiative and Health Canada’s Forward Regulatory Plan, provide opportunities for Canadians and stakeholders to become involved in decision-making processes, including the development of the regulatory process. For example:
|
| Modernize communications about Health Canada’s role as a regulator |
Continue to acquire, develop and improve the tools, processes and resources needed to effectively communicate with, and engage, Canadians on Health Canada’s digital platforms, including Canada.ca and Health Canada social media channels. For example:
|
| Advance regulatory modernization initiatives |
Continue to implement the Department’s regulatory modernization commitments described in the Health and Biosciences: Targeted Regulatory Review – Regulatory Roadmap, to make Canada’s science-based regulatory system more agile and internationally aligned. Through these initiatives, including the introduction of agile regulations for drugs and medical devices, Health Canada will enhance its ability to provide comprehensive drug oversight, ensure safety, and maintain position as a top-tier regulator. For example:
|
- Risk: Health Canada’s ability to help protect the health of Canadians may be weakened due to the complexity and fragility of the global supply chain, the rapid pace of innovation, and increasing e-commerce from global sources.
| Strengthen oversight |
Develop strategies and tools to strengthen market surveillance and oversight of emerging products and supply shortage. For example:
|
|---|---|
| Collaborate internationally |
Collaborate with international regulatory organizations and align where appropriate with foreign regulators. For example:
|
| Address changing business models in the supply chain |
Strengthen oversight of foreign sites involved in the manufacturing of health products. For example:
|
Planned results for Core Responsibility 2: Health Protection and Promotion
The following table shows, for Core Responsibility 2: Health Protection and Promotion, the planned results, the result indicators, the targets and the target dates for 2023–24, and the actual results for the three most recent fiscal years for which actual results are available.
| Departmental Result Indicators | Target | Date to achieve target | Actual Results |
|---|---|---|---|
| Percentage of human new drug decisions issued within service standardsTable 3 note 1 (Baseline: 88% 82% for pharmaceuticals; 100% for biologic and radiopharmaceuticals established in 2017-18) |
At least 93% | March 31, 2024 | 2019-20: 96.0% 2020-21: 100.0% 2021-22: 99.8% |
| Percentage of Risk Management Plan reviews for new drug decisions completed within service standards (Baseline: 94% established in 2018-19) |
At least 90% | March 31, 2024 | 2019-20: 93% 2020-21: 94% 2021-22: 90% |
| Percentage of domestic drug companies deemed to be compliant with manufacturing requirements under the Food and Drugs Act and associated Regulations (Baseline: 94% established in 2018-19) |
At least 90%Table 3 note 2 | March 31, 2024 | 2019-20: 96.0% 2020-21: 99.7% 2021-22: 97.0% |
Table 3 Notes
|
|||
| Departmental Result Indicators | Target | Date to achieve target | Actual Results |
|---|---|---|---|
| Percentage of domestic consumer product recalls communicated to Canadians in a timely manner (Baseline: 86% in 2016-17) |
At least 90% | March 31, 2024 | 2019-20: 86% 2020-21: 86% 2021-22: 83% |
| Percentage of actions taken in a timely manner to protect the health of Canadians from substances found to be a risk to human health (Baseline: 85% in 2016-17) |
Exactly 100% | March 31, 2024 | 2019-20: 100% 2020-21: 100% 2021-22: 100% |
| Percentage of pre-market pesticide submission reviews that are completed within service standards (Baseline: 95% in 2019-20) |
At least 90% | March 31, 2024 | 2019-20: 95% 2020-21: 93% 2021-22: 96% |
| Departmental Result Indicators | Target | Date to achieve target | Actual Results |
|---|---|---|---|
| Percentage of Canadians (aged 15+) who are current cigarette smokers (Baseline: 16% in 2017) |
At most 5% | March 31, 2035 | 2019-20: 17.8% 2020-21: 13.0%Table 4 note 1 2021-22: 12.0%Table 4 note 2 |
| Percentage of youth (grades 10-12) who report frequent (daily to weekly) cannabis use in the past 30 days (Baseline: 9.2% in 2018-19) |
At most 9.2% | March 31, 2025 | 2019-20: 9.2% 2020-21: 9.2%Table 4 note 3 2021-22: N/ATable 4 note 3 |
| Percentage of Canadians who use dietary guidance provided by Health Canada (Baseline: 94% established in 2018-19) |
At least 50% | March 31, 2024 | 2019-20: 47% 2020-21: 47% 2021-22: 44%Table 4 note 4 |
Table 4 Notes
|
|||
Financial, human resources and performance information for Health Canada’s Program Inventory is available on GC InfoBase.
Planned budgetary financial resources (dollars) for Core Responsibility 2: Health Protection and Promotion
The following table shows, for Core Responsibility 2: Health Protection and Promotion, budgetary spending for 2023–24, as well as planned spending for that year and for each of the next two fiscal years.
| 2023-24 budgetary spending (as indicated in Main Estimates) | 2023-24 Planned spending |
2024-25 Planned spending |
2025-26 Planned spending |
|---|---|---|---|
| 834,117,084 | 834,117,084 | 682,081,158 | 532,719,255 |
|
Note: The decrease in planned spending in 2024-25 is mainly due to funding level decreases for addressing the opioid crisis and the Substance Use and Addictions Program; as well as to the expiry of budgetary authorities for continuing Canada’s chemical management regime; strengthening the capacity and transparency of the pesticide review process; bringing innovation to regulations; and ensuring the ongoing integrity of the Public Service Occupational Health Program.
The decrease in planned spending in 2025-26 is mainly due to the expiry of budgetary authorities for the renewal of the federal framework for the legalization and regulation of cannabis in Canada; and addressing the opioid crisis and substance use.
The Department would have to request funding for these initiatives for future years. |
|||
Planned Human resources (full-time equivalents) for Core Responsibility 2: Health Protection and Promotion
The following table shows, in fulltime equivalents, the human resources the department will need to fulfill this core responsibility for 2023–24 and for each of the next two fiscal years.
| 2023-24 Planned full-time equivalents |
2024-25 Planned full-time equivalents |
2025-26 Planned full-time equivalents |
|---|---|---|
| 6,371 | 5,949 | 5,144 |
|
Note: The decrease in planned FTEs in 2024-25 is mainly due to the expiry of budgetary authorities for continuing Canada’s chemical management regime; strengthening the capacity and transparency of the pesticide review process; bringing innovation to regulations; ensuring the ongoing integrity of the Public Service Occupational Health Program; and enhancing the federal response to the opioid crisis in Canada.
The decrease in planned FTEs in 2025-26 is mainly due to the expiry of budgetary authorities for the renewal of the federal framework for the legalization and regulation of cannabis in Canada; and addressing the opioid crisis and substance use.
The Department would have to request funding for these initiatives for future years. |
||
Financial, human resources and performance information for Health Canada’s Program Inventory is available on GC InfoBase.
Internal Services
Description
Internal Services are those groups of related activities and resources that the federal government considers to be services in support of Programs and/or required to meet corporate obligations of an organization. Internal Services refers to the activities and resources of the 10 distinct services that support Program delivery in the organization, regardless of the Internal Services delivery model in a department.
The 10 service categories are: Management and Oversight Services; Communications Services; Legal Services; Human Resources Management Services; Financial Management Services; Information Management Services; Information Technology Services; Real Property Management Services; Materiel Management Services; and Acquisition Management Services.
Planning Highlights
Health Canada’s greatest strength is an engaged, empowered and well-equipped workforce with employees who have the competencies (including science and regulatory skill sets), tools and opportunities to succeed in the pursuit of excellence in program and service delivery.
Did you know?
In 2022, Health Canada was selected as one of Canada’s Top 100 Employers, as one of Canada’s Best Diversity Employers, as one of Canada’s Top Employers of Young People, and as a National Capital Region’s Top Employer, with recognition for the Department’s efforts to build and sustain a healthy and inclusive workplace.
The Clerk of the Privy Council noted in his letter to the Prime Minister in the Twenty-Ninth Annual Report on the Public Service of Canada the collective hard work of public servants during a period characterized by the GOC’s response to the COVID-19 pandemic and Canada’s confrontation with racism and intolerance. This period has presented the public service with many opportunities to serve in different ways and to adapt while responding to complex emerging challenges. Health Canada continues to support the government-wide goals of Public Service Renewal through initiatives that foster inclusivity, agility and resilience.
Health Canada provided pandemic-response leadership in occupational and employee mental health throughout 2022-23, which included supporting workplace re-entry, both within the Health Portfolio and across the Federal Public Service. In 2023-24, the Department will continue to leverage lessons learned to promote and facilitate post-pandemic mental health resources to support and encourage evidence-based occupational health practices.
What’s new?
In 2023-24, as part of Health Canada’s response to the Call to Action on Anti-Racism, Equity, and Inclusion in the Federal Public Service, the Department will take steps to eliminate racism or bias in science workplaces (laboratories and offices), methodologies and the application of science findings through policies and programs. Health Canada will also update its Framework for Science and Research Excellence to reference applying an anti-racism lens to science and research processes.
In 2023-24, the Department will also focus on the following priorities: building a healthy, diverse and inclusive workforce; enabling a safe and productive workforce with access to modern tools and facilities; and communicating with Canadians.
Building a healthy, diverse and inclusive workforce
Health Canada remains committed to its ongoing response to the Clerk’s Call to Action on Anti-Racism, Equity, and Inclusion in the Federal Public Service, with the goal of ensuring a work environment that is free of racism and discrimination, and where all employees feel safe and are treated with respect, dignity and fairness. These values are the foundation of who we are, what we do, and how we carry out our work. Priorities include:
- Addressing systemic racism, harassment, and discrimination towards employment equity groups via the Leadership Council on Diversity and Inclusion; the Inclusion, Diversity, Equity and Accessibility Employee Networks Collaboration Forum; Employee Networks; and through implementing the recommendations from the Inclusive Staffing Working Group and Diverse Selection Board initiative;
- Continuing to implement the findings of the Internal Anti-Racism Listening Sessions as they relate to recruitment, onboarding and retention;
- Continuing to implement the Equitable Access to Language Training Program to support equitable access to language training for employment equity groups;
- Implementing the Mentorship Plus program to encourage leadership development, with emphasis on supporting members of underrepresented groups who aspire to leadership and executive positions;
- Implementing initiatives to meet the commitments set out in the Department’s first Accessibility Plan;
- Developing clear performance measures to improve accessibility with a focus on: employment; built environment and the workplace; communications and technologies; program design and service delivery; and culture change.
The Department will work to attract and retain a diverse, bilingual and representative workforce within a healthy, equitable and inclusive workplace by:
- Continuing to offer training to employees to help prevent violence and harassment in the workplace;
- Including bias and barrier-free practices for recruitment, onboarding, and retention within the Multi-Year Diversity and Employment Equity Plan for 2022-25;
- Increasing recruitment and representation of persons with disabilities in support of the Accessibility Strategy for the Public Service of Canada;
- Ensuring that accessibility, diversity and inclusivity are key considerations in the planning and development of supports for the workforce, work, and workplace, and that Health Canada hires its share of the GOC commitment to hire 5,000 persons with disabilities by 2025;
- Implementing Emotional Intelligence coaching and training to support the development of our leaders;
- Implementing the Department’s Official Languages Action Plan for 2022-25.
Through the Centre for Ombuds, Resolution and Ethics (CORE), Health Canada will nurture a respectful, diverse, and inclusive work environment by providing a safe space where employees, at all levels, can raise and discuss work-related concerns and find options, resources, and tools to address them. To support organizational health and well-being within the Department, CORE will raise awareness of systemic issues to those with the authority to act and will foster collaborative approaches to managing workplace conflicts. CORE will also guide the modernization of the Departmental Code of Values and Ethics and promote it to all employees.
Enabling a safe and productive workforce with access to modern tools and facilities
Health Canada will continue to collaborate across the Health Portfolio to encourage and support the modernization and security of the workforce. Employees will continue to have access to modern tools and facilities, including those that meet the requirements for accessibility as per the National Accommodation Strategy. Further, the Department will continue to support employees through mental health resources such as the Mental Health Toolkit, LifeSpeak and the Employee Assistance Program.
Health Canada’s Chief Data Office champions digital-data transformation by leveraging data as an asset to support evidence-based decision-making and enhance the efficiency and effectiveness of programs and services. Driven by the Department’s Data Strategy and the Open Science Action Plan, the Office will help create an environment of data access and collaboration to unlock the power of data.
The Department will continue to explore and experiment with new technologies such as artificial intelligence and remain agile in its delivery of essential programs and services. This will enable the Department to leverage digitally enabled solutions and provide health programs and services to Canadians in an effective manner.
Other priorities in 2023-24 include:
- Providing IT systems and collaboration tools that support a hybrid work environment, allowing employees to perform their duties both remotely and in the office in a seamless manner;
- Enhancing cyber-security awareness by providing ongoing cyber-security awareness programs and training to support the new hybrid working environment;
- Establishing a digital strategy to support the modernization of both existing and new services provided by the Department;
- Continuing to transform the Department’s financial, materiel, and grants and contributions management systems to align with the GOC’s Digital Comptrollership Program.
The Department is also committed to renewing its Real Property Portfolio Strategy while continuing to implement its long-term capital plan and to participate in Labs Canada to support modern and collaborative laboratories.
Communicating with Canadians
Health Canada will continue to provide Canadians with inclusive, timely and evidence-based information they need to take action on personal and collective health and safety. Communication platforms and services will be effectively leveraged to provide trusted, accurate, accessible and culturally appropriate information that puts all Canadians first in its design and functionality. The Department will also ensure visibility and awareness of Departmental programs and Ministerial priorities, such as the new interim Canada Dental Benefit and the development of the longer-term Canada-wide dental care program.
Health Canada will also maintain its leadership role in delivering evidence-based and innovative public education and awareness initiatives and work with P/Ts and other stakeholders to inform Canadians on priority topics such as: opioids and other controlled substances; addiction; overdose; safer supply; decriminalization of personal possession; tobacco and vaping; cannabis; chronic pain; mental health; regulatory compliance and enforcement; drug shortages; healthy eating; food and product safety; pesticides; regulation and authorization of health products; and environmental health, including climate change.
Key Risk(s) for Internal Services
- Risk: Health Canada’s ability to deliver on its mandate effectively may be at risk due to challenges in maintaining a high-performing, bilingual, and diverse and agile workforce within a healthy workplace.
| Support workplace wellness initiatives |
Invest in initiatives to foster a healthy and safe workplace. For example:
|
|---|---|
| Promote diversity, bilingualism and inclusion |
Encourage diversity, bilingualism and inclusion. For example:
|
| Attract and retain skilled and talented employees |
Maintain a high-performing workforce with the appropriate skills and competencies. For example:
|
- Risk: Health Canada’s ability to deliver its programs and services may be at risk due to the Department’s aging physical and IT infrastructure, deferred maintenance, limited funding, limited data analytics capacity, and challenges in safeguarding IT assets from cyberattacks.
| Continue to update IT and lab infrastructure |
Equip employees with modern, enhanced and secure infrastructure. For example:
|
|---|---|
| Promoting digital transformation |
Leverage data as an asset to enhance effective and efficacy of programs and services by:
|
| Promote training and awareness |
Ensure Department vigilance and raise employee awareness. For example:
|
| Strengthen oversight |
Implement oversight strategies and foster a security culture remotely and onsite. For example:
|
Planning for Contracts Awarded to Indigenous Businesses
Health Canada is committed to meeting the mandatory minimum target to award contracts to Indigenous Businesses as per the Government of Canada’s commitment that a mandatory minimum target of 5% of the total value of contracts is awarded to Indigenous businesses annually. To facilitate this, the Department is continuing to champion change and awareness by developing guidance and tools, collaborating with other government departments to leverage expertise and best practices, and monitoring progress. Program managers and contracting authorities will be further supported through training and learning opportunities in 2023-24.
The following table shows in % the actual, forecasted and planned value for the target.
| 5% reporting field description | 2021-22 actual % achieved | 2022-23 forecasted % target | 2023-24 planned % target |
|---|---|---|---|
| Total percentage of contracts with Indigenous businesses | N/A | N/A | 5% |
Planned budgetary financial resources (dollars) for internal services
The following table shows, for internal services, budgetary spending for 2023–24, as well as planned spending for that year and for each of the next two fiscal years.
| 2023-24 budgetary spending (as indicated in Main Estimates) |
2023-24 Planned spending |
2024-25 Planned spending |
2025-26 Planned spending |
|---|---|---|---|
| 307,931,603 | 307,931,603 | 281,938,421 | 270,294,735 |
|
Note: The decrease in planned spending in 2024-25 is mainly due to the expiry of budgetary authorities for bringing innovation to regulations; the implementation of greening initiatives; continuing Canada’s chemical management regime; and strengthening the capacity and transparency of the pesticide review process; as well as funding level decreases to implement the interim Canada Dental Benefit Plan.
The decrease in planned spending in 2025-26 is mainly due to the expiry of budgetary authorities for the renewal of the federal framework for the legalization and regulation of cannabis in Canada; and to implement the interim Canada Dental Benefit Plan.
The Department would have to request funding for these initiatives for future years. |
|||
Planned human resources (full-time equivalents) for internal services
The following table shows, in full time equivalents, the human resources the department will need to carry out its internal services for 2023–24 and for each of the next two fiscal years.
| 2023-24 Planned full-time equivalents |
2024-25 Planned full-time equivalents |
2025-26 Planned full-time equivalents |
|---|---|---|
| 2,056 | 1,994 | 1,923 |
|
Note: The decrease in planned FTEs in 2024-25 is mainly due to the expiry of budgetary authorities for continuing Canada’s chemical management regime; bringing innovation to regulations; and strengthening the capacity and transparency of the pesticide review process; as well as funding level decreases to implement the interim Canada Dental Benefit Plan.
The decrease in planned FTEs in 2025-26 is mainly due to the expiry of budgetary authorities for the renewal of the federal framework for the legalization and regulation of cannabis in Canada; and to implement the interim Canada Dental Benefit Plan.
The Department would have to request funding for these initiatives for future years. |
||
Sex- and Gender-Based Analysis Plus
In 2023-24, Health Canada will continue to implement its Sex- and Gender-Based Analysis Plus (SGBA Plus) Action Plan, providing a framework to strengthen the systemic integration of sex, gender and diversity considerations into its initiatives, departmental culture and operations.
The Action Plan outlines strategies across three pillars: accountability, knowledge and evidence, and capacity and expertise. Activities include:
- Increasing governance, accountability and transparency in the integration of SGBA Plus in the Department’s decision-making;
- Enhancing Departmental knowledge and capacity to apply SGBA Plus using an intersectional lens and a deeper understanding of systemic issues to advance equity, diversity and inclusion in the work of the Department;
- Collaborating with internal and external partners to strengthen the Department’s sex-, gender- and diversity-related evidence base and expertise;
- Enabling the collection and use of disaggregated data for rigour in intersectional analysis;
- Enhancing communications, guidelines, tools and resources with clarity on SGBA Plus and intersectionality.
Aligning with the Plan’s priorities, Health Canada will continue to leverage its SGBA Plus Integration Network to provide tools to facilitate, promote and support the systematic integration of SGBA Plus into its activities and organizational processes.
The Department will also continue to provide employee training in various SGBA Plus-related areas to increase awareness of how SGBA Plus could and should be integrated in the development of policies and guidelines and apply SGBA Plus to project management and risk communications. Integrating SGBA Plus and Indigenous lenses in the development and delivery of programs will serve to protect the health of all Canadians.
Other Departmental SGBA Plus initiatives planned for 2023-24
The planned initiatives described below will increase the extent to which the needs of diverse groups are considered in the Department’s internal operations and in its service to the public.
For example, Health Canada will improve the Department’s Employee Assistance Program by:
- Continuing recruitment efforts to improve alignment of the counsellor network with public service demographics;
- Expanding the collection of demographic data to include specific sub-groups within the “Racialized” category to enhance how disaggregated data is collected and evaluated;
- Continuing promotion of live Chat and other digital wellness resources through social media and other means (presentations, information sessions, website);
- Continuing promotion of the diversity-themed organizational services (training and other wellness supports) available for managers and their teams.
Supporting a universal and equitable publicly funded healthcare system
The CHA provides an important opportunity for considering diverse needs. By monitoring respective operations of P/Ts health care insurance plans, Health Canada advises the Minister of Health on possible non-compliance with the CHA. Under the Act, all insured health care services should be provided free of any barriers to access, including discrimination on the basis of race, to all eligible residents of Canada, including Indigenous peoples.
Monitoring efforts have identified both real and potential compliance issues for specific SGBA Plus cohorts, including pregnant persons and individuals seeking abortions. When issues of non-compliance are suspected, the Department will encourage P/Ts to share detailed information, including the number of patients affected and amount of patient charges. The data will be used to understand the size and scope of the issues and potentially inform decisions on deduction amounts to P/T Canada Health Transfer payments when issues cannot be resolved through collaboration.
What’s new?
Health Canada will incorporate SGBA Plus in its Privacy Risk assessments to assess the privacy impacts of programs, initiatives, and activities on diverse groups of women, men, and gender diverse people.
In 2023-24, Health Canada will update and use performance measurement tools to monitor and analyze progress made by recipients of funding from the Official Languages Health Program. These tools will expand the capacity of both Health Canada and recipients to monitor, collect, analyze and report (annually or as required) on progress towards achieving expected results by gender/sex and diversity. This will help recipients track progress towards incorporating these considerations to reach target/at-risk populations and ensure adequate implementation of measures that include SGBA Plus.
Similarly, recipients of Palliative Care funding will be encouraged to consider the intersection of diverse factors that affect access to and the experience of providing and receiving palliative care. This includes but is not limited to sex, gender, official language status and Indigenous status. Recipients will also continue to report on sex, gender, and where possible, other sociodemographic indicators. Improving access to palliative care for underserved populations (including children, communities experiencing racism, 2SLGBTQI+ communities, etc.) continues to be a priority, and programming to address this is in development and underway. Health Canada will engage with these communities to ensure their unique needs and experiences are represented.
Strengthening sex- and gender-related evidence and practices
With a view to ensuring rigour in intersectional analysis and mitigating health risks, Health Canada also remains committed to advancing multiple projects to ensure collection and analysis of disaggregated data through various means, as outlined below:
Guidance and regulations
- Amending regulations to require the submission of disaggregated data in New Drug Submissions and Supplements to New Drug Submissions;
- Continuing to update medical device guidance documents to include SGBA Plus considerations as it relates to Clinical Evidence Requirements, with details on sex disaggregated data;
- Promoting a new, modern, user-centric food guide recipe gallery that will include culturally-diverse recipes and articles relevant to a broader range of people living in Canada;
- Increasing the impact of public education campaigns by considering gender and other diversity-related factors when selecting a target audience, drafting key messages, developing strategies and tactics, producing creative concepts and determining strategies based on media consumption habits.
Health risks and safety
- Ongoing collaboration with CIHR and Women and Gender Equality Canada to explore the feasibility of establishing a Canadian breast implant registry, designed to improve patient notification following the identification of a safety concern;
- Establishing a Science Research Integrity Network SGBA Plus Working Group to improve the integration of sex, gender and diversity analysis into Health Canada’s Decision-Making Framework for identifying, assessing, and managing health risks;
- Systematically integrating SGBA Plus into regulatory initiatives for chemicals to strengthen and more rigorously apply SGBA Plus considerations within its chemicals management activities;
- Continuing to require that manufacturers provide stratified data based on patient characteristics (for example, sex, pregnancy, age and ethnicity) in Monthly Safety Summary Reports and Risk Management Plans to allow Health Canada reviewers to formally document SGBA plus analyses.
More information on SGBA Plus is available in the Gender-Based Analysis Plus supplementary information table.
The United Nations 2030 Agenda for Sustainable Development and UN Sustainable Development Goals (SDGs)
The 2030 Agenda for Sustainable Development, adopted by Canada and all 193 United Nations member states in 2015, is a global framework centered around an ambitious set of 17 Sustainable Development Goals (SDGs), covering the interconnected economic, social, and environmental dimensions of sustainable development. It aims to eradicate poverty, protect the planet, and ensure prosperity by the year 2030. Central to the 2030 Agenda is the theme “to leave no one behind.” In response to this, the GOC established three cross-cutting objectives to support the SDGs:
- Leaving no-one behind (i.e., disproportionately affected populations);
- Advancing Reconciliation with Indigenous Peoples;
- Ensuring Canada’s international efforts support the advancement of the SDGs.
Canada’s commitment to the 2030 Agenda
A National Strategy on the 2030 Agenda was published in February 2021 and defines Canada’s commitment to advancing progress on the SDGs through widespread collaborative engagement and action. A Federal Implementation Plan has also been developed to articulate how the GOC will contribute to the advancement of the National Strategy at the federal level and how it will report on progress to Canadians. Progress will be measured through the Canadian Indicator Framework(CIF) established in 2021.
Defining Health Canada’s support for the SDGs
Health Canada protects the health of Canadians, and promotes overall health and well-being, considering a broad range of personal, social, economic and environmental factors that contribute to individual and population health. As such, the Department’s domestic contribution to the 2030 Agenda directly supports the following SDGs through ongoing policies, programs and initiatives:
Fostering sustainable healthcare systems
The Department supports SDG 3 promoting the good health and well-being of Canadians through healthcare system and service delivery innovation, including e-prescribing and access to virtual care; improving patient safety and quality care; and strengthening Canada’s healthcare systems with a focus on improving the capacity to protect marginalized populations.
Health Canada supports initiatives that improve access to appropriate and effective health care services (including MAID; home, community and palliative care; and cancer care). The Department also supports health human resources (for example, recruiting nurses) and work towards a national universal system of pharmacare.
The GOC introduced Canada’s first Canada-wide dental benefit to improve access to dental care services for children under 12, since oral health is a major contributor to overall health and well-being and evidence has shown that socioeconomic factors, including income, education, employment, are determinants of oral health.
This work supports the Canadian Indicator Framework (CIF) Ambition “Canadians have healthy and satisfying lives.”
Community-based substance use and mental health services
The Department is expanding access to community-based substance use services for children and youth and spreading evidence-based models of community mental health care and culturally appropriate interventions that are integrated with primary health services. Health Canada is also expanding the availability of integrated community-based mental health and substance use services for people with complex health needs and setting national standards for access to mental health and substance use services to improve access to support the needs of Canadians (SDG 3).
This work supports the CIF Ambition “Canadians have healthy and satisfying lives” and related CIF Indicators 3.7.1 and 3.12.1.
Healthier living supports
The Department promotes healthier living by helping prevent and minimize substance use harms through the Canadian Drugs and Substances Strategy, the Substance Use and Addictions Program, and Canada’s Tobacco Strategy. These supports include educating Canadians on the risks of tobacco, opioids, cannabis use, vaping and alcohol by funding initiatives across Canada aimed at reaching communities at greatest risk, or who may face barriers accessing services (SDG 3). This supports the CIF Ambitions “Canadians adopt healthy behaviours” and “Canada prevents causes of premature death” as well as the related CIF indicators 3.2.1, 3.4.1, 3.12.1, and 3.13.1.
Health Canada provides information to Canadians on healthy eating that considers equity and is inclusive of Canada’s diverse population. The Department also continues to collaborate internationally on food safety and nutrition standards through establishing policies and regulations related to the safety and nutritional quality of all food sold in Canada. This supports the CIF Ambition “Canadians Adopt Healthy Behaviours” (SDG 3) and the related CIF indicator 3.1.1. The Department also ensures access to safe, effective and quality health products by introducing innovative and agile regulatory measures (SDG 3).
Drinking water guidelines and indoor air pollution
Health Canada develops and updates Canadian drinking water guidelines and guidance documents in partnership with the P/Ts and other federal departments. These guidelines are used by jurisdictions in Canada as the basis for establishing drinking water quality requirements for all Canadians (SDG 6). This supports the CIF Ambition “Canadians have access to drinking water and use it in a sustainable manner.
In addition, Health Canada’s research, tools and science-based information helps to inform Canadians about the health effects of ambient and indoor air pollution (SDG 11). This supports the CIF Ambition “Canadians live in healthy, accessible, and sustainable cities and communities” and the related CIF indicator 11.3.1.
Supporting the safe management of chemicals
To protect the environment and Canadians from harmful substances, Health Canada continues its collaboration with partner departments to support the safe and sustainable use of pesticide products through the application of current science. In addition, the Department will continue to work with Environment and Climate Change Canada and other partners to deliver Canada’s Chemicals Management Plan (CMP). The CMP assesses and manages risks to human health and the environment posed by chemical substances that can be found in everyday items, such as food and food products, consumer products, cosmetics, drugs, drinking water and industrial releases (SDG 12). This supports the CIF Ambition “Canadians consume in a sustainable manner.” Under the umbrella of the CMP, Health Canada is also taking steps to enable more meaningful partnerships and place greater focus on the priorities of Indigenous Peoples.
Health Canada’s initiatives to build climate change resilience
Did you know?
Health Canada is co-chairing a Working Group lead by the World Health Organization on Climate Resilient Health Systems, with a focus on building climate resilience and adaptation to current, emerging and future health impacts and threats of climate change.
Health Canada’s ongoing work to build capacity through initiatives to support the health sector in preparing for and adapting to the impacts of climate change (SDG 13) supports the CIF Ambition “Canadians are well-equipped and resilient to face the effects of climate change.”
The Department is also working on protecting Canadians from extreme heat and on improving heat resiliency across Canada. In addition, Health Canada is advancing the Health and Wellbeing component of Canada’s first National Adaptation Strategy in collaboration with Environment and Climate Change Canada. Health Canada will invest to expand the Protecting Canadians from Extreme Heat Program to provide the best available guidance and resources to Canadians experiencing extreme heat and the HealthADAPT Program to support partners across Canada in creating climate-resilient health systems.
More information on specific areas of work that support SDGs and the related cross-cutting objectives is available in the United Nations 2030 Agenda and the Sustainable Development Goals supplementary information table.
Innovation
Innovation is critical to Health Canada’s ability to meet its mandate in the face of rapidly evolving science, post-pandemic recovery, and the changing demands and expectations of Canadians.
Innovation activities continue to be supported by the Department in order to explore, test, and compare the effects and impacts of policies and interventions. This process will inform decision-making and improve outcomes for Canadians.
Health Canada has seen increased innovation and experimentation work in the areas of enterprise digital and data transformation, Robotic Process Automation, Artificial Intelligence (AI), and policy design.
The Department’s Solutions Fund (the Fund) empowers employees to create and lead innovation and experimentation projects that will improve how Health Canada provides services to Canadians and brings new ideas and efficiencies as to how it does business. The aim of the Fund is to improve services to Canadians, improve departmental operations and functionality, and deliver greater value to taxpayers.
The Department will continue to invest in innovation and experimentation projects in 2023-24, including:
- Project Apollo – Testing the effectiveness of digital game-based learning as an educational tool to increase awareness of potential environmental health risks (air quality, radon, and chemicals) among Canadian youth. This project will integrate the latest behavioural change theories and principles into the game design and testing methodology.
- Project Cognit.IO – Testing a human-centric, AI-assisted engine to support and augment the accuracy, consistency, and speed of assessing complex natural health products. This technology could help address the large volume of product applications.
- Project Heart – Exploring methods to help strengthen Health Canada’s ability to engage equitably and inclusively with a wide range of people with lived experience with a health related issue to make better-informed policy and program decisions. Final reports will include ideas on how to improve engagement and set the stage for testing these improvements.
- Project D.A.T.A. (Data Annotation Training sets for Artificial Intelligent Tools) – Testing the potential of crowdsourcing and data governance at the enterprise level by developing a natural language processing training set to structure and collect data from scientific literature. The project will also seek collaboration to leverage the training set for use in AI tools.
What’s new?
In 2023-24, progress will be measured on Department-wide familiarity with elements of Health Canada’s Framework for Science and Research Excellence. This Framework provides a clear, structured guide for thinking and talking about science to support an Open Science approach throughout the Department.
Other examples of innovation and experimentation initiatives that will begin or continue in 2023-24 include:
- Project Citizen Science – Exploring the feasibility and infrastructure needed to support broad uptake of citizen science in health research using a user-centered approach between federal scientists and volunteers from the public. This work supports the Department’s Open Science Action Plan, Health Canada’s Framework for Science and Research Excellence and Canada’s 2022-24 National Action Plan on Open Government.
- Project Kelpie – piloting a social media monitoring data stream service to use real-time data analysis to monitor social media posts that promote vaping products to youth.
- Project GenomicsCompTox Cloud Computing – exploring the use of cloud computing services to perform analytical pipelines, automations and applications services for genomics and computational toxicology research. Performance and cost benchmarks will be compared to the existing on-premises hardware infrastructure.
- The Chemicals Management Plan Contribution Program – focusing efforts on more inclusive decision-making and reconciliation through the Indigenous Funding Stream. This project aims to help further understanding about how traditional knowledge can complement scientific information.
Health Canada officials will also assess how the Department can leverage its participation in the GOC’s Innovative Solutions Canada Challenge program to support innovation to help meet the Department’s priorities. One existing Health Canada project to develop a cost-effective and reliable method to identify pathogens to support the appropriate use of microbial mixtures will conclude in 2023-24.
Planned spending and human resources
This section provides an overview of the department’s planned spending and human resources for the next three fiscal years and compares planned spending for 2023-24 with actual spending for the current year and the previous year.
Planned spending
Departmental spending 2020-21 to 2025-26
The following graph presents planned spending (voted and statutory expenditures) over time.
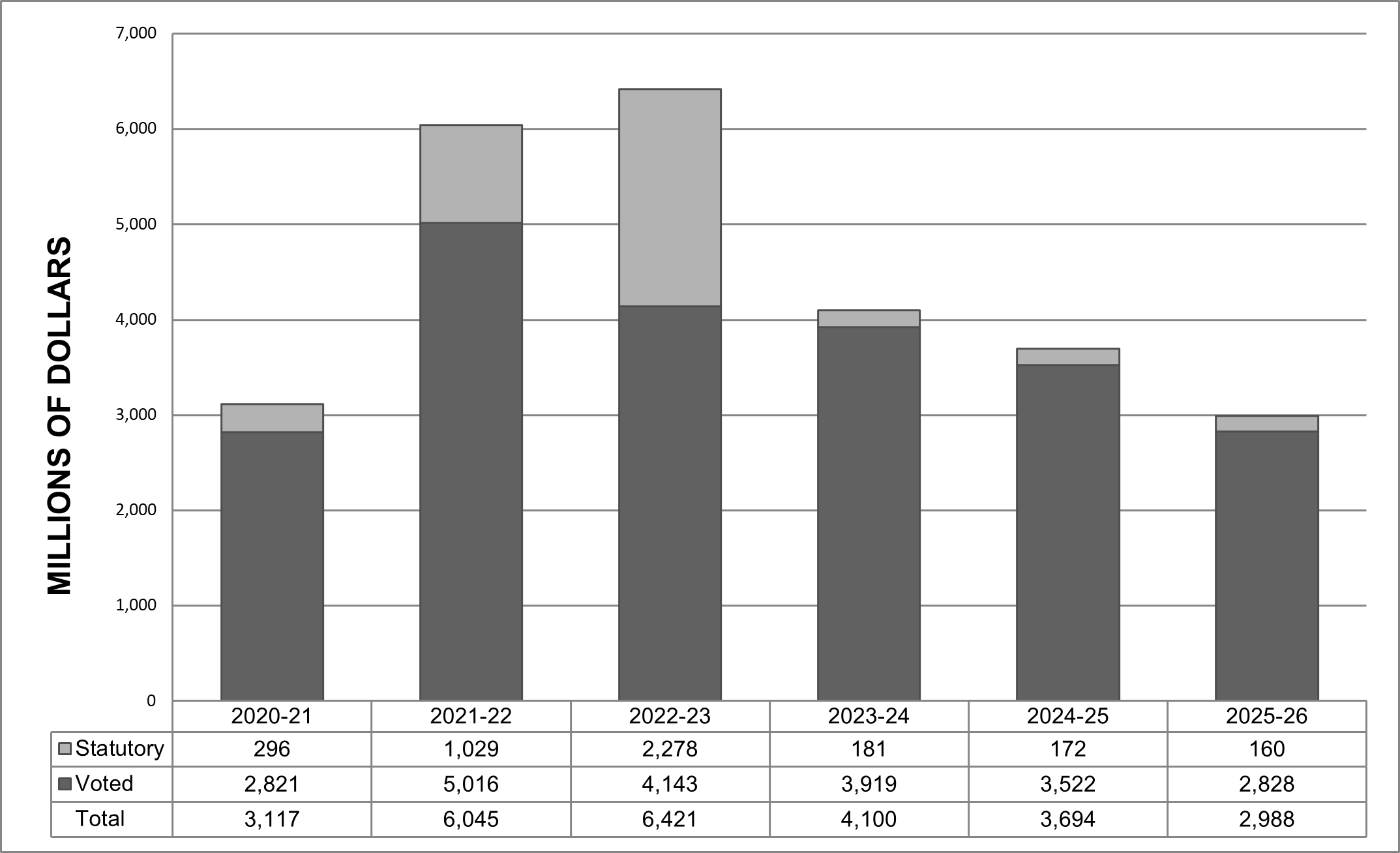
Figure 1 – Text description
The figure illustrates Health Canada’s spending trend from fiscal year 2020-21 to fiscal year 2025-26 where spending, in millions of dollars, is shown on the vertical axis and time period, in fiscal years, is shown on the horizontal axis.
Health Canada’s actual spending for fiscal year 2020-21: $3,117 million (Voted: $2,821 million, Statutory: $296 million); and 2021-22: $6,045 million (Voted: $5,016 million, Statutory: $1,029 million).
Health Canada’s forecast spending for fiscal year 2022-23: $6,421 million (Voted: $4,143 million, Statutory: $2,278 million).
Health Canada’s planned spending for fiscal year 2023-24: $4,100 million (Voted: $3,919 million, Statutory: $181 million); 2024-25: $3,694 million (Voted: $3,522 million, Statutory: $172 million); and 2025-26: $2,988 million (Voted: $2,828 million, Statutory: $160 million).
Budgetary planning summary for Core Responsibilities and Internal Services (dollars)
The following table shows information on spending for each of Health Canada’s Core Responsibilities and to Internal Services for 2023-24 and other relevant fiscal years.
| Core Responsibilities and Internal Services | 2020-21 expenditures |
2021-22 expenditures |
2022-23 forecast spending |
2023-24 budgetary spending (as indicated in Main Estimates) |
2023-24 planned spending |
2024-25 planned spending |
2025-26 planned spending |
|---|---|---|---|---|---|---|---|
| Core Responsibility 1: Health Care Systems | 1,987,223,947 | 4,744,300,568 | 5,081,736,929 | 2,958,177,598 | 2,958,177,598 | 2,730,077,364 | 2,184,574,828 |
| Core Responsibility 2: Health Protection and Promotion | 660,580,250 | 787,250,023 | 897,139,083 | 834,117,084 | 834,117,084 | 682,081,158 | 532,719,255 |
| Subtotal | 2,647,804,197 | 5,531,550,591 | 5,978,876,012 | 3,792,294,682 | 3,792,294,682 | 3,412,158,522 | 2,717,294,083 |
| Internal Services | 468,848,746 | 513,234,110 | 442,196,066 | 307,931,603 | 307,931,603 | 281,938,421 | 270,294,735 |
| Total | 3,116,652,943 | 6,044,784,701 | 6,421,072,078 | 4,100,226,285 | 4,100,226,285 | 3,694,096,943 | 2,987,588,818 |
|
Note: The 2020–21 to 2022–23 fiscal years total expenditures and forecast spending include all parliamentary appropriation sources: Main Estimates, Supplementary Estimates, and funding from various Treasury Board votes. For the 2023–24 to 2025–26 fiscal years, total planned spending does not include funding through Supplementary Estimates and carry forward adjustments.
The increase in actual expenditures in 2021–22 and 2022–23 is mainly due to Health Canada’s response to the COVID-19 pandemic.
The decrease in planned spending in 2023-24 is mainly due to statutory funding received in 2022-23 for the procurement of additional rapid test kits and Canada dental benefits; as well as the expiry of budgetary authorities in 2022–23 for emergency measures related to the pandemic; the continued response to the COVID19 pandemic; Canada Health Infoway; and Territorial Health Investment Fund.
The decrease in planned spending in 2024–25 is mainly due to funding level decreases to implement the interim Canada Dental Benefit Plan; and addressing the opioid crisis and the Substance Use and Addictions Program; as well as the expiry of budgetary spending authorities for mental health supports and services; continuing Canada’s chemical management regime; the Canadian Drug Agency Transition Office; enhancing the federal response to the opioid crisis in Canada; supporting access to sexual and reproductive health care information and services; and strengthening the capacity and transparency of the pesticide review process.
The decrease in planned spending in 2025–26 is mainly due to the expiry of budgetary spending authorities for developing a national strategy for high-cost drugs for rare diseases; the renewal of the federal framework for the legalization and regulation of cannabis in Canada; addressing the opioid crisis and substance use; and to implement the interim Canada Dental Benefit Plan.
For the expiry of budgetary spending authorities, the Department would have to request funding for these initiatives for future years. |
|||||||
Planned human resources
The following table shows information on human resources, in full-time equivalents (FTEs), for each of Health Canada’s core responsibilities and for its internal services for 2023–24 and the other relevant years.
| Core Responsibilities and Internal Services | 2020-21 actual FTEs |
2021-22 actual FTEs |
2022-23 forecast FTEs |
2023-24 planned FTEs |
2024-25 planned FTEs |
2025-26 planned FTEs |
|---|---|---|---|---|---|---|
| Core Responsibility 1: Health Care Systems | 247 | 428 | 342 | 385 | 299 | 272 |
| Core Responsibility 2: Health Protection and Promotion | 6,036 | 6,527 | 6,560 | 6,371 | 5,949 | 5,144 |
| Subtotal | 6,283 | 6,955 | 6,902 | 6,756 | 6,248 | 5,416 |
| Internal Services | 2,344 | 2,573 | 2,017 | 2,056 | 1,994 | 1,923 |
| Total | 8,627 | 9,528 | 8,919 | 8,812 | 8,242 | 7,339 |
|
Note: The 2020–21 and 2021–22 fiscal years full-time equivalents (FTEs) are based on actual expenditures on personnel. The 2022–23 fiscal year is based on total authorities from all parliamentary appropriation sources: Main Estimates and Supplementary Estimates. For the 2023–24 to 2025–26 fiscal years, total FTEs do not include FTEs funded through Supplementary Estimates and carry forward adjustments. The calculation of the planned FTE figures is based on programs using their full revenue authority.
The increase in actual FTEs for 2021–22 and 2022–23 is mainly due to Health Canada’s response to the COVID-19 pandemic.
The decrease in planned FTEs in 2023–24 is mainly due to the expiry of budgetary authorities in 2022–23 for the transition to new impact assessment and regulatory processes; the Canadian Agriculture Partnership framework; for the creation of a critical drug reserve in collaboration with P/Ts to support access to drugs; as well as investments in cannabis public education, awareness, research and hental health.
The decrease in planned FTEs in 2024–25 is mainly due to the expiry of budgetary authorities for continuing Canada’s chemical management regime; strengthening the capacity and transparency of the pesticide review process; bringing innovation to regulations; ensuring the ongoing integrity of the Public Service Occupational Health Program; the Canadian Drug Agency Transition Office; enhancing the federal response to the opioid crisis in Canada; as well as funding level decreases to implement the interim Canada Dental Benefit Plan.
The decrease in planned FTEs in 2025–26 is mainly due to the expiry of budgetary authorities in 2024–25 for the renewal of the federal framework for the legalization and regulation of cannabis in Canada; developing a national strategy for high-cost drugs for rare diseases; addressing the opioid crisis and substance use; and to implement the interim Canada Dental Benefit Plan.
For the expiry of budgetary spending authorities, the Department would have to request funding for these initiatives for future years. |
||||||
Estimates by vote
Information on the Health Canada’s organizational appropriations is available in the 2023-24 Main Estimates.
Future-oriented condensed statement of operations
The future oriented condensed statement of operations provides an overview of Health Canada’s operations for 2022-23 to 2023-24.
The forecast and planned amounts in this statement of operations were prepared on an accrual basis. The forecast and planned amounts presented in other sections of the Departmental Plan were prepared on an expenditure basis. Amounts may therefore differ.
A more detailed future-oriented statement of operations and associated notes, including a reconciliation of the net cost of operations to the requested authorities, are available on Health Canada’s website.
| Financial information | 2022-23 forecast results |
2023-24 planned results |
Difference (2023-24 planned results minus 2022-23 forecast results) |
|---|---|---|---|
| Total expenses | 4,925,655,995 | 4,433,856,613 | (491,799,382) |
| Total revenues | 273,082,452 | 284,850,298 | 11,767,846 |
| Net cost of operations before government funding and transfers | 4,652,573,543 | 4,149,006,315 | (503,567,228) |
|
Health Canada is projecting $4,433.9 million in expenses based on 2023-24 Main Estimates and accrual information. This amount does not include future supplementary estimates. It represents a decrease of $491.8 million from 2022-23 forecast results.
The decrease in planned results is mainly due to statutory authority reported in 2022-23 for the procurement of COVID-19 tests. This decrease is partially offset by the following increases:
The 2023-24 planned expenses by core responsibility are as follows:
Health Canada receives most of its funding through annual Parliamentary appropriations. Health Canada’s revenue is generated by programs that support the above-noted core responsibilities. Health Canada projects total revenues in 2023-24 to be $284.9 million, representing an increase of $11.8 million from 2022-23 projections.
The 2023-24 main sources of revenues by type are as follows:
|
|||
Corporate information
Organizational profile
Appropriate Ministers: The Honourable Jean-Yves Duclos, P.C., M.P.
and The Honourable Dr. Carolyn Bennett, P.C., M.P.
Institutional Head: Dr. Stephen Lucas
Ministerial portfolio: Health
Enabling instrument[s]: Assisted Human Reproduction Act, Canada Consumer Product Safety Act, Canada Health Act, Cannabis Act, Controlled Drugs and Substances Act, Department of Health Act, Dental Benefit Act, Food and Drugs Act, Hazardous Materials Information Review Act, Hazardous Products Act, Pest Control Products Act, Radiation Emitting Devices Act, Tobacco and Vaping Products Act
List of Acts and Regulations
Year of incorporation / commencement: 1913
Raison d’être, mandate and role
Raison d’être, mandate and role: who we are and what we do is available on the Health Canada website.
For more information on the Department’s organizational mandate letter commitments, see the mandate letters for the Minister of Health and Minister of Mental Health and Addictions and Associate Minister of Health.
Operating context
Information on the operating context is available on the Health Canada website.
Reporting framework
Health Canada’s approved Departmental Results Framework and Program Inventory for 2023-24 are as follows.
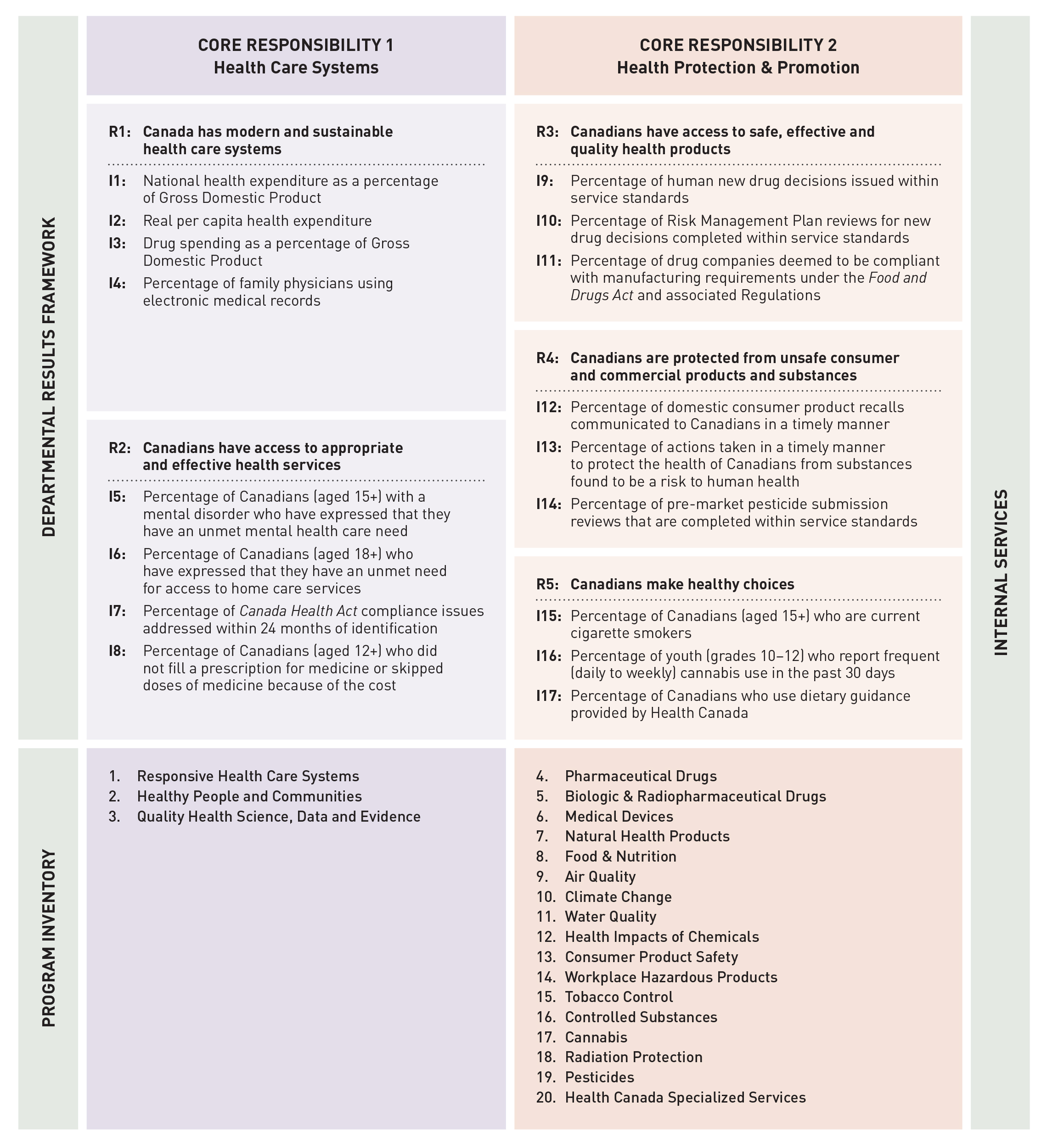
Figure 2 – Text description
Legend:
R: Result
I: Indicator
The figure illustrates Health Canada’s approved Departmental Results Framework and Program Inventory for 2023-24.
The Departmental results Framework groups Health Canada’s Core Responsibilities into two categories, all supported by Internal Services. These categories are (1) Health Care Systems and (2) Health Protection and Promotion, each of which are delivered through multiple programs in the Program Inventory. Each core responsibility has departmental results and several indicators associated with it.
Departmental Results Framework
Core Responsibility 1: Health Care Systems
- R1: Canada has modern and sustainable health care systems
- I1: National health expenditure as a percentage of Gross Domestic Product
- I2: Real per capita health expenditure
- I3: Drug spending as a percentage of Gross Domestic Product
- I4: Percentage of family physicians using electronic medical records
- R2: Canadians have access to appropriate and effective health services
- I5: Percentage of Canadians (aged 15+) with a mental disorder who have expressed that they have an unmet mental health care need
- I6: Percentage of Canadians (aged 18+) who have expressed that they have an unmet need for access to home care services
- I7: Percentage of Canada Health Act compliance issues addressed within 24 months of identification
- I8: Percentage of Canadians (aged 12+) who did not fill a prescription for medicine or skipped doses of medicine because of the cost
Program Inventory under Core Responsibility 1 (from one – three) as follows:
- Responsive Health Care Systems
- Healthy People and Communities
- Quality Health Science, Data and Evidence
Core Responsibility 2: Health Protection and Promotion
- R3: Canadians have access to safe, effective and quality health products
- I9: Percentage of human new drug decisions issued within service standards
- I10: Percentage of Risk Management Plan reviews for new drug decisions completed within service standards
- I11: Percentage of domestic drug companies deemed to be compliant with manufacturing requirements under the Food and Drugs Act and associated regulations
- R4: Canadians are protected from unsafe consumer and commercial products and substances
- I12: Percentage of domestic consumer product recalls communicated to Canadians in a timely manner
- I13: Percentage of actions taken in a timely manner to protect the health of Canadians from substances found to be a risk to human health
- I14: Percentage of pre-market pesticide submission reviews that are completed within service standards
- R5: Canadians make healthy choices
- I15: Percentage of Canadians (aged 15+) who are current cigarette smokers
- I16: Percentage of youth (grades 10-12) who report frequent (daily to weekly) cannabis use in the past 30 days
- I17: Percentage of Canadians who use dietary guidance provided by Health Canada
Program Inventory under Core Responsibility 2 (from four-20) as follows:
- Pharmaceutical Drugs
- Biologic & Radiopharmaceutical Drugs
- Medical Devices
- Natural Health Products
- Food & Nutrition
- Air Quality
- Climate Change
- Water Quality
- Health Impacts of Chemicals
- Consumer Product Safety
- Workplace Hazardous Products
- Tobacco Control
- Controlled Substances
- Cannabis
- Radiation Protection
- Pesticides
- Health Canada Specialized Services
Internal Services
Changes to the approved reporting framework since 2022–23
The programs under Core Responsibility 1 have been significantly restructured in 2023-24. Three broad new programs (Responsive Health Care Systems; Healthy People and Communities; and Quality Health Science, Data and Evidence) have replaced the previous 15 outlined in 2022-23.
While some of the original 15 programs (like the “Canada Health Act Program”) were moved in their entirety into one of the new programs, others were distributed across 2 or more of the new programs. For example, the organs and tissue component of the “Organs, Tissues and Blood Program” was moved to the new “Responsive Health Care Systems Program” while the blood component was moved to the “Quality Health Science, Data and Evidence Program.”
These 3 new programs are more inclusive and accommodate a broader set of activities. They also emphasize Health Canada’s own policy work and research, which was under-represented in the previous structure. Lastly, they reduce the focus on Grants and Contributions funding and thus reduce the reliance on the results of specific funding recipients.
| Structure | 2023-24 | 2022-23 | Change | Reason for Change |
|---|---|---|---|---|
| CORE RESPONSIBILITY 1 | Health Care Systems | Health Care Systems | No change | |
| PROGRAM | Responsive Health Care Systems | Health Care Systems Analysis and Policy (bilateral agreements only); Access, Affordability, and Appropriate Use of Drugs and Medical Devices; Home, Community and Palliative Care; Mental Health (bilateral agreement only); Digital Health; Canada Health Act; Cancer Control; Organs, Tissues and Blood (organ and tissue donation only); Territorial Health Investment Fund (Ended in 2022-23) | New program | See above |
| PROGRAM | Healthy People and Communities | Health Care Systems Analysis and Policy (all but bilateral agreements); Mental Health (all but bilateral agreement); Promoting Minority Official Languages in the Health Care Systems; Thalidomide | New program | See above |
| PROGRAM | Quality Health Science, Data and Evidence | Health Information; Organs, Tissue and Blood (blood only); Brain Research | New program | See above |
| CORE RESPONSIBILITY 2 | Health Protection and Promotion | Health Protection and Promotion | No change | Not applicable |
| PROGRAM | Pharmaceutical Drugs | Pharmaceutical Drugs | No change | Not applicable |
| PROGRAM | Biologic & Radiopharmaceutical Drugs | Biologic & Radiopharmaceutical Drugs | No change | Not applicable |
| PROGRAM | Medical Devices | Medical Devices | No change | Not applicable |
| PROGRAM | Natural Health Products | Natural Health Products | No change | Not applicable |
| PROGRAM | Food & Nutrition | Food & Nutrition | No change | Not applicable |
| PROGRAM | Air Quality | Air Quality | No change | Not applicable |
| PROGRAM | Climate Change | Climate Change | No change | Not applicable |
| PROGRAM | Water Quality | Water Quality | No change | Not applicable |
| PROGRAM | Health Impacts of Chemicals | Health Impacts of Chemicals | No change | Not applicable |
| PROGRAM | Consumer Product Safety | Consumer Product Safety | No change | Not applicable |
| PROGRAM | Workplace Hazardous Products | Workplace Hazardous Products | No change | Not applicable |
| PROGRAM | Tobacco Control | Tobacco Control | No change | Not applicable |
| PROGRAM | Controlled Substances | Controlled Substances | No change | Not applicable |
| PROGRAM | Cannabis | Cannabis | No change | Not applicable |
| PROGRAM | Radiation Protection | Radiation Protection | No change | Not applicable |
| PROGRAM | Pesticides | Pesticides | No change | Not applicable |
| PROGRAM | Health Canada Specialized Services | Health Canada Specialized Services | No change | Not applicable |
Supporting information on the Program Inventory
Supporting information on planned expenditures, human resources, and results related to Health Canada’s Program Inventory is available on GC InfoBase.
Supplementary information tables
The following supplementary information tables are available on Health Canada’s website:
- Details on transfer payment programs
- Gender-based analysis plus
- Horizontal initiatives
- United Nations 2030 Agenda and the Sustainable Development Goals
Federal tax expenditures
Health Canada’s Departmental Plan does not include information on tax expenditures.
Tax expenditures are the responsibility of the Minister of Finance, and the Department of Finance Canada publishes cost estimates and projections for government¬ wide tax expenditures each year in the Report on Federal Tax Expenditures. This report provides detailed information on tax expenditures, including objectives, historical background and references to related federal spending programs, as well as evaluations, research papers and gender-based analysis plus.
Organizational contact information
Serena Francis
Assistant Deputy Minister / Chief Financial Officer
Health Canada
Assistant Deputy Minister Office
70 Colombine Driveway, Tunney’s Pasture Ottawa, Ontario K1A 0K9
Telephone: 613-295-8651
[email protected]
Appendix: Definitions
- appropriation (crédit)
- Any authority of Parliament to pay money out of the Consolidated Revenue Fund.
- budgetary expenditures (dépenses budgétaires)
- Operating and capital expenditures; transfer payments to other levels of government, organizations or individuals; and payments to Crown corporations.
- core responsibility (responsabilité essentielle)
- An enduring function or role performed by a department. The intentions of the department with respect to a core responsibility are reflected in one or more related departmental results that the department seeks to contribute to or influence.
- Departmental Plan (plan ministériel)
- A document that sets out a department’s priorities, programs, expected results and associated resource requirements, covering a three-year period beginning with the year indicated in the title of the report. Departmental Plans are tabled in Parliament each spring.
- departmental result (résultat ministériel)
- A change that a department seeks to influence. A departmental result is often outside departments’ immediate control, but it should be influenced by program-level outcomes.
- departmental result indicator (indicateur de résultat ministériel)
- A factor or variable that provides a valid and reliable means to measure or describe progress on a departmental result.
- departmental results framework (cadre ministériel des résultats)
- A framework that consists of the department’s core responsibilities, departmental results and departmental result indicators.
- Departmental Results Report (rapport sur les résultats ministériels)
- A report on a department’s actual performance in a fiscal year against its plans, priorities and expected results set out in its Departmental Plan for that year. Departmental Results Reports are usually tabled in Parliament each fall.
- full- time equivalent (équivalent temps plein)
- A measure of the extent to which an employee represents a full person year charge against a departmental budget. Full-time equivalents are calculated as a ratio of assigned hours of work to scheduled hours of work. Scheduled hours of work are set out in collective agreements.
- gender-based analysis plus (GBA Plus) (analyse comparative entre les sexes plus [ACS Plus])
- An analytical tool used to support the development of responsive and inclusive policies, programs and other initiatives. GBA Plus is a process for understanding who is impacted by the issue or opportunity being addressed by the initiative; identifying how the initiative could be tailored to meet diverse needs of the people most impacted; and anticipating and mitigating any barriers to accessing or benefitting from the initiative. GBA Plus is an intersectional analysis that goes beyond biological (sex) and socio-cultural (gender) differences to consider other factors, such as age, disability, education, ethnicity, economic status, geography, language, race, religion, and sexual orientation.
- government-wide priorities (priorités pangouvernementales)
- For the purpose of the 2023–24 Departmental Plan, government-wide priorities are the high-level themes outlining the Government’s agenda in the 2021 Speech from the Throne: building a healthier today and tomorrow; growing a more resilient economy; bolder climate action; fighter harder for safer communities; standing up for diversity and inclusion; moving faster on the path to reconciliation and fighting for a secure, just, and equitable world.
- high impact innovation (innovation à impact élevé)
- High impact innovation varies per organizational context. In some cases, it could mean trying something significantly new or different from the status quo. In other cases, it might mean making incremental improvements that relate to a high-spending area or addressing problems faced by a significant number of Canadians or public servants.
- horizontal initiative (initiative horizontale)
- An initiative in which two or more federal organizations are given funding to pursue a shared outcome, often linked to a government priority.
- non budgetary expenditures (dépenses non budgétaires)
- Net outlays and receipts related to loans, investments and advances, which change the composition of the financial assets of the Government of Canada.
- performance (rendement)
- What an organization did with its resources to achieve its results, how well those results compare to what the organization intended to achieve, and how well lessons learned have been identified.
- plan (plan)
- The articulation of strategic choices, which provides information on how an organization intends to achieve its priorities and associated results. Generally, a plan will explain the logic behind the strategies chosen and tend to focus on actions that lead up to the expected result.
- planned spending (dépenses prévues)
- For Departmental Plans and Departmental Results Reports, planned spending refers to those amounts presented in the Main Estimates.
A department is expected to be aware of the authorities that it has sought and received. The determination of planned spending is a departmental responsibility, and departments must be able to defend the expenditure and accrual numbers presented in their Departmental Plans and Departmental Results Reports. - program (programme)
- Individual or groups of services, activities or combinations thereof that are managed together within a department and that focus on a specific set of outputs, outcomes or service levels.
- program inventory (répertoire des programmes)
- An inventory of a department’s programs that describes how resources are organized to carry out the department’s core responsibilities and achieve its planned results.
- result (résultat)
- An external consequence attributed, in part, to an organization, policy, program or initiative. Results are not within the control of a single organization, policy, program or initiative; instead, they are within the area of the organization’s influence.
- statutory expenditures (dépenses législatives)
- Expenditures that Parliament has approved through legislation other than appropriation acts. The legislation sets out the purpose of the expenditures and the terms and conditions under which they may be made.
- target (cible)
- A measurable performance or success level that an organization, program or initiative plans to achieve within a specified time period. Targets can be either quantitative or qualitative.
- voted expenditures (dépenses votées)
- Expenditures that Parliament approves annually through an Appropriation Act. The vote wording becomes the governing conditions under which these expenditures may be made.
Footnotes
- Footnote 1
-
The Government of Canada recognizes First Nations, Inuit and the Métis Nation, as the Indigenous Peoples of Canada, consisting of distinct, rights-bearing communities with their own histories, including with the Crown. The work of forming renewed relationships based on the implementation of rights, respect, co-operation, and partnership must reflect the unique interests, priorities and circumstances of each People. Health care policy development needs to recognize these distinctions.
link


